Abstract
Background
Potentially inappropriate prescribing (PIP) is one of the main risk factors for adverse drug events (ADEs) in older people.
Purpose
This systematic literature review aims to determine prevalence and type of PIP in community-dwelling older people across Europe, as well as identifying risk factors for PIP.
Methods
The PubMed and Web of Science database were searched systematically for relevant manuscripts (January 1, 2000–December 31, 2014). Manuscripts were included if the study design was observational, the study participants were community-dwelling older patients in Europe, and if a published screening method for PIP was used. Studies that focused on specific pathologies or that focused on merely one inappropriate prescribing issue were excluded. Data analysis was performed using R statistics.
Results
Fifty-two manuscripts were included, describing 82 different sample screenings with an estimated overall PIP prevalence of 22.6 % (CI 19.2–26.7 %; range 0.0–98.0 %). Ten of the sample screenings were based on the Beers 1997 criteria, 19 on the Beers 2003 criteria, 14 on STOPP criteria (2008 version), 8 on START-criteria (2008 version), and 7 on the PRISCUS list. The 24 remaining sample screenings were carried out using compilations of screening methods or used country-specific lists such as the Laroche criteria. It appears that only PIP prevalence calculated from insurance data significantly differs from the other data collection method categories. Furthermore, risk factors most often positively associated with PIP prevalence were polypharmacy, poor functional status, and depression. Drug groups most often involved in PIP were anxiolytics (ATC-code: N05B), antidepressants (N06A), and nonsteroidal anti-inflammatory and anti-rheumatic products (M01A).
Conclusion
PIP prevalence in European community-dwelling older adults is high and depends partially on the data collection method used. Polypharmacy, poor functional status, and depression were identified as the most common risk factors for PIP.
Similar content being viewed by others

Introduction
Over the past years, the proportion of the European population aged 65 years and over has been increasing [1]. Aging is often associated with a growing number of chronic diseases and hence polypharmacy, which increases the risk for adverse drug events (ADEs) [2, 3], drug-related hospitalizations, and related costs [2, 4]. A recent systematic review reported an ADE prevalence up to 23 % for older adults in ambulatory care with preventability rates up to 53 % [3].
Previous studies have identified potentially inappropriate prescribing (PIP) as one of the main risk factors for ADEs in older adults [5–9]. PIP is defined as the prescribing of medication that could introduce a significant risk of an ADE, in particular when there is an equally or more effective alternative with lower risk available [10, 11]. PIP encompasses three main categories: over-, under-, and misprescribing (e.g., inappropriate dose or duration) [12]. Both explicit (criteria-based) and implicit (judgment-based) screening methods were developed and used to detect PIP [13–16]. Studies using these tools clearly demonstrated that PIP prevalence is high and that an early detection may indeed prevent hospitalizations and improve health outcomes [10, 17–19]. Research to detect and reduce PIP was initially mainly situated in hospital and nursing home settings as one of the strategies to prevent and lower the prevalence of ADEs [20–22]. Over the past decade, however, PIP screening in primary care received increasing attention among health-care workers because detecting and tackling PIP at that point in the health-care system could be more (cost-)effective [10, 19].
In 2012, Opondo et al. published the first systematic review on PIP in the primary care setting [10]. Eight of the 19 included studies were carried out in Europe, describing an overall median PIP rate of 19.1 % (range 2.9–38.5 %). However, this review only included studies that reported on ‘unconditionally inappropriate medication prescriptions.’ Studies investigating drug-drug interactions, drug-disease interactions, or other types of PIP were excluded. In addition, risk factors for PIP have not yet been reviewed. This information is however needed to enable the design of well-defined targeted interventions to improve the quality of prescribing for older adults. Furthermore, since the publication of the systematic review of Opondo et al. [10], several new studies in the European ambulatory setting have emerged, which have contributed to a better characterization of the contemporary field [23–26].
In order to improve quality of care for community-dwelling older adults, it is necessary to determine the magnitude, nature, and relevance of PIP in Europe. Only then, straightforward and targeted management plans for patient groups at risk can be developed to tackle the most problematic PIPs in primary care. The aim of this systematic review is (1) to synthesize observational research on PIP prevalence in community-dwelling older adults in Europe, (2) to present an overview of the risk factors mostly described in association with PIP, and (3) to summarize the drugs or drug groups most often involved in PIP.
Methods
Searches
We searched the PubMed and Thomson Reuters Web of Science™ databases for relevant manuscripts from January 1, 2000 to December 31, 2014. The search strategy contained terms and combinations related to older patients, medications, (in)appropriateness, ambulatory care, outpatient care, or community-dwelling patients, including the MeSH terms “Aged” and “Inappropriate prescribing” as shown in the Online Supplement. The final literature search was performed on December 31, 2014. The database search was completed with a manual search of the reference lists of included articles (i.e., “snowballing”). The quality of the systematic review was supported by the use of the PRISMA guidelines [27].
Eligibility criteria
Manuscripts were eligible for inclusion if they met the following criteria: (1) study design was observational, (2) study participants were community-dwelling older patients (65 years and older) in Europe, and (3) a published screening method, either implicit or explicit for PIP was used. Manuscripts could be published in English, French, Dutch, German, or Spanish. Studies that focused on specific pathologies (e.g., patients with dementia) or that focused on merely one inappropriate prescribing issue (e.g., benzodiazepine use) were excluded in addition to manuscripts that not specifically mentioned a focus on the primary care setting or the older age group.
Study selection
Duplicate manuscripts were removed after exporting search results to endnote (Thomson Reuters, Times Square, New York, NY, USA). Subsequently, two reviewers (ET and EM) independently screened the title, abstract, and full text of the retrieved manuscripts for eligibility. Each manuscript showing uncertainty regarding inclusion criteria was discussed until consensus about inclusion in the following selection round was reached.
Data collection, synthesis, and analysis
Data concerning country, study period, inclusion criteria, used data collection method, and used screening method were collected from the selected manuscripts. Additional extracted data included sample size, mean (SD), age of the screened population, mean (SD), or median (IQR) number of drugs taken (as presented in the original manuscript and therefore sometimes including OTC-drugs), and PIP prevalence (reported as the percentage of patients or prescriptions with at least one PIP). When repeated measurements were reported, we included only the most recent rate of PIP. When data could not be retrieved from the published manuscript, we contacted the corresponding author to request for additional information. If the corresponding author did not reply, a reminder e-mail was sent 1 month later. The study quality (“risk of bias”) of the included studies was evaluated by using a slightly adapted quality assessment scale from the Cochrane Collaboration group, including the following domains: study participation, data collection, screening method used, outcome measurement, and statistical analysis. All studies were judged having a low or moderate risk of bias.
We estimated an overall prevalence with its 95 % confidence interval (CI), using a random effects model with random intercepts. Prevalence data were modeled on a logit scale using sample size as a weighting factor. Likewise, a conditional prevalence with its 95 % CI was estimated for the most used PIP screening methods (Beers, STOPP, START, and PRISCUS), each method of data collection ((1) face-to-face interview, (2) prescribing, dispensing, or primary health database, (3) insurance data, (4) questionnaire, (5) medical record, or (6) combinations), and their interactions, using a (saturated) random effects model with fixed effects. For this analysis, all Beers analyses and combinations of data collection methods were collapsed into single categories. Analyses were carried out using R® (R Foundation for Statistical Computing, Vienna, Austria).
Subsequently, we selected those manuscripts that assessed risk factors associated with PIP and extracted the results as mentioned in the original manuscript. For the factor ‘polypharmacy,’ different groups used other definitions (varying from the use of ≥4 up to ≥7 drugs), which were merged for reasons of comparability. Finally, we selected those manuscripts that provided detailed information about PIP prevalence for specific drugs or drug groups and summarized the information. The ten drugs or drug groups most frequently associated with PIP were extracted as originally mentioned in the included manuscript and classified according to the Anatomical Therapeutic Chemical (ATC) classification system (3rd level) (e.g., ‘M01AB05—diclofenac’ was classified as ‘M01A—anti-inflammatory and antirheumatic products, nonsteroids’ or ‘B01AC—platelet aggregation inhibitors’ as ‘B01A—antithrombotic agents’) [28]. Due to the heterogeneity of the methodologies applied in the included studies, the overview of risk factors and drugs or drug groups most frequently associated with PIP is merely reported in a descriptive way.
Results
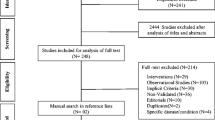
We identified 1375 manuscripts and screened 1138 titles and 154 abstracts for eligibility after duplicate removal. We screened the full text of 62 manuscripts and excluded 23. Thirteen manuscripts were added via manual search of the references. Our final sample comprised 52 manuscripts reporting on 82 sample screenings of PIP in community-dwelling older adults in Europe (Fig. 1). These studies were performed in 23 different European countries. The eventual list of included manuscripts is presented in Table 1, arranged by country and year of publication.
PIP prevalence
The overall estimated weighted PIP prevalence was 22.6 % (CI 19.5–26.7 %). PIP prevalence ranged from 0.0 to 98.0 %, with sample sizes varying from 50 to 1,019,491 patients. A large heterogeneity and inconsistency in study design (data collection methods) and outcome measures (screening methods used) was however observed.
Eighteen of the 52 included manuscripts used more than one screening method to assess PIP prevalence, resulting in 82 different sample screenings. Nineteen of the sample screenings were based on Beers 2003 criteria [11], 10 on the Beers 1997 criteria [31], 14 on STOPP criteria (2008 version) [55], 8 on START criteria (2008 version) [55], and 7 on the PRISCUS list [16]. Twenty-two sample screenings were carried out using compilations of the previously mentioned lists or used country-specific lists such as the Laroche criteria [42], the improving prescribing in the elderly tool (IPET), [53], or NORGEP-criteria [64]. Only two sample screenings used the medication appropriateness index (MAI) but accounted however for the two highest mentioned prevalence rates (84 and 98 %) [34, 80]. In only 14 of the 82 sample screenings (17 %), the complete original screening method was maintained. In all other sample screenings (n = 68), the screening method was adapted.
Accordingly, large differences were seen in the method of data collection. Of the 52 included manuscripts, 16 collected data via patient interviews (30 sample screenings). Eleven studies used insurance data (16 sample screenings) while 11 manuscripts used another specific type of database (dispensing, prescribing, or primary care database—13 sample screenings). Four studies used medical records (9 sample screenings), 4 used a questionnaire, either filled in by a general practitioner or patient (5 sample screenings), and 6 used a combination of data sources (9 sample screenings).
To estimate an overall conditional prevalence for different methods of data collection, different screening methods, and possible interactions, 59 sample screenings were included in the random effects model (parameter estimates from the model are presented in Table OS1 of the Online Supplement). Only the data collection method proved to be a significant predictor in the model, and in addition, it appears that only PIP prevalence calculated from insurance data significantly differs from the other data collection method categories (p < .05).
Factors associated with PIP
Twenty-seven of the 52 included manuscripts evaluated factors associated with overuse and misuse without taking underuse into consideration. Seventeen used multivariate logistic regression analyses, 8 used a bivariate logistic regression, and 2 used a univariate logistic regression. The studies evaluated a total of 24 different risk factors. All risk factors, evaluated in at least three studies, are presented in Table 2. Polypharmacy, advanced age, and female gender were most often taken into account; however, only polypharmacy showed a consistent positive association with PIP. Factors that were less often taken into account but showed a repeated positive association in multiple studies with PIP were presence of depression, moderate self-rated health quality, a low functional status, or a poor economic status.
Only 2 manuscripts reviewed factors associated with underuse of medication, taking into account polypharmacy, advanced age, and female gender in the analyses. One study found a positive association between underuse of medication and polypharmacy and the other between underuse and advanced age. One study found a negative association between underuse of medication and female gender [2, 3, 24].
Drugs most frequently involved in PIP
Forty of the 52 included manuscripts mentioned detailed drug information on 53 sample screenings (Table OS2 of the online supplement).
Forty-seven of those 53 sample screenings used a screening method to detect overuse or misuse. The most frequently overused or misused drugs were (1) anxiolytics (N05B), (2) antidepressants (N06A), and (3) nonsteroidal anti-inflammatory and anti-rheumatic products (M01A). PIP dependent on an underlying diagnosis is part of misuse of drug. However, only 15 of the 47 sample screenings used a screening method that—additional to PIPs independent of underlying diagnosis—also included PIP dependent on confirmed diagnosis (e.g., different STOPP criteria, the second part of the Beers list). Therefore, no PIPs dependent on underlying diagnoses are mentioned in Table OS2. We believe however that this information is of importance, and therefore, we performed a sub-analysis to detect these PIPs and reported them separately in a subsection of Table OS2. Eleven of the 15 sample screenings taking into account underlying diagnoses used the STOPP criteria and 4, the second part of the Beers list. Most frequent were (1) the long-term use of NSAIDs (>3 months) in mild osteoarthritis, (2) the use of calcium channel blockers in chronic constipation, and (3) the use of noncardioselective β-blockers in patients with COPD.
The six remaining sample screenings used a tool to detect underuse of medication, and all made use of START-criteria. When considering the ten most prevalent items of each screening, a total of 17 different START-criteria were detected. The START criterion most often mentioned by the included sample screenings was the omission of antiplatelet therapy in diabetes mellitus with co-existing major cardiovascular risk factors. This criterion was detected in all of the six sample screenings. An overview of the other most prevalent underused drugs detected by START-criteria is given in Table OS2 of the online supplement.
Discussion
In this systematic literature review, we evaluated the prevalence of PIP in community-dwelling older adults across Europe. Our review included studies evaluating all types of PIP (overuse, misuse, and underuse) and included all studies irrespective of the fact that they presented detailed information on the specific PIP items or used a specific type of data collection. Fifty-two manuscripts were selected, describing 82 different sample screenings with an estimated weighted overall PIP prevalence of 22.6 % (CI 19.5–26.7 %).
PIP prevalence
Consistent with the reviews performed by Aparasu et al. [21] and Opondo et al. [10] (both mainly USA-based), our review found that about one in five older patients in Europe is exposed to PIP. This suggests that the possible inappropriateness of prescribing in Europe and the USA is comparable. To obtain a contemporary image of the problem, we only included manuscripts published after 2000. It seems though that the overall PIP rate has not substantially decreased since the previous reviews despite considerable attention in the scientific literature and increasing research on this topic (22 manuscripts published between 2000 and 2009 compared to 30 between 2010 and 2014). Three other reviews, by Shade et al. [18], Hill-Taylor et al. [17], and Guaraldo et al. [87], did not calculate an overall PIP prevalence. Our estimated mean falls however within the ranges observed in these studies (22.7–74 % [17], 21.4–79 % [17], and 11.5–62.5 % [87]).
The wide range in observed PIP prevalence probably relates to the wide diversity of screening methods used and the sometimes extensive adaptations to these screening methods. In 83 % of the sample screenings, the original screening method was adapted; either because it was not compatible with the applied data collection method (e.g., STOPP/START on insurance data with no clinical data detected significantly lower prevalence of 6 % compared to 30 % when used on medical record—Table OS1) or because it didn’t fully match the European setting (e.g., Beers criteria). Using such adapted screening methods seems contra-intuitive, hampers interpretation of the data, and probably leads to an underestimation of the true PIP prevalence. If limited clinical data are available, the use of the GheOP [3], S-tool [88], or the Matanovic criteria [33] may offer a better approach since they both present a comprehensive protocol that screens for overuse, misuse, and underuse; are adapted to the European setting; and do not require clinical data or confirmed diagnoses.
Moreover, as underuse, overuse, and misuse are all types of PIP [12], it is surprising that in multiple research, PIP prevalence is separately given for START and STOPP criteria [23, 69, 72]. Presenting the prevalence as the proportion of patients with at least one START or STOPP criterion might represent a more accurate image of PIP. Additionally, different data collection methods, differences in quality of prescribing across geographical regions, or the status of medication review practices in the European countries could furthermore also have contributed to the reported wide range [89, 90].
Despite the impossibility of comparing the individual sample screenings, the information presented in the sample screenings did give an interesting insight in the way data collection methods or used screening methods influence PIP prevalence (see Table OS1). Although not significant, there is a trend that the combination of data collection methods leads to higher prevalence. As well, the two screenings that used an implicit screening method—the MAI— showed the highest PIP prevalence. It will be interesting to see whether the new version of STOPP/START [91], where some implicit criteria were added, will follow this trend and lead to higher prevalence rates.
Factors associated with PIP
Many research studies showed that advanced age and polypharmacy are important and independent risk factors for the presence of PIPs and ADEs [17, 87]. It was however unclear whether other factors are also of significance and could help to further target patient groups at risk for PIPs and ADEs. The present evaluation of risk factors showed that polypharmacy, a low functional status, depression, a moderate self-rated health quality, poor economic situation, a high comorbidity score, and reduced cognition are most often positively associated with a higher risk for PIP. It was however remarkable that from those studies that evaluated the association between PIP and advanced age, only in about half a positive association was found [17, 87].
Drugs most frequently involved in PIP
In concordance with previous findings [10], we observed that the use of anxiolytics, hypnotics, and sedatives are most often involved in PIP. In addition, antidepressants (such as amitriptyline and doxepin), NSAIDs, and antithrombotic agents (such as ticlopidine and dipyridamole) are also often involved in PIP. One drug group that was not mentioned in previous systematic research as substantially associated with PIP, but highly present in this review, is the “antihistamines for systemic use” group.
In our review, we additionally focused on PIPs depending on a confirmed diagnosis and underuse because until now, only little systematic research on these types of PIP has been performed. It appears that NSAIDs are most often interfering with underlying diseases such as peptic ulcer and moderate or severe hypertension. In contrast to other research [92–94], interactions with chronic heart failure, dementia, and renal impairment are not often detected in our review. This discrepancy could be explained by the fact that many of the research included in the current review used data collection methods without confirmed diagnoses as three were solely dispensing databases, five were based on patient interviews, and seven (partially) on medical records.
Considering underuse, the results of our review are in line with a previous review performed by Hill-Taylor et al., reviewing studies that used START criteria [17]. Besides the underuse of calcium/vitamin D supplements and statin therapy, we observed that antiplatelet therapy and metformin are often omitted in diabetes mellitus as well as β-blockers in chronic stable angina.
Strengths and limitations
In order to obtain as much available research as possible, more than one electronic database was used and literature was supplemented by manually checking reference lists of all included manuscripts. Compared to previously published reviews [10, 87], this systematic review gives a much more global overview of PIP, including underuse, drug-disease interactions, and drug groups most often associated with PIP. In addition, the review specifically focuses on the European setting which was not the focus of any other previous research [10, 17, 18, 21, 87]. Furthermore, this is the first review summarizing all research regarding risk factors for PIP. By limiting the time period and including all European studies, we attempted to provide a contemporary and country-specific overview of PIP, offering a point of reference for countries in which PIP is still poorly characterized. Nevertheless, several limitations remain when interpreting the findings of this systematic review. Comparing results from the included manuscripts was difficult due to use of different inclusion criteria, different screening methods, and inconsistent adaptations of these tools. Additionally, differences in health-care settings and countries may also have impacted PIP prevalence.
Conclusion
This systematic review shows that PIP prevalence in community-dwelling older adults in Europe remains high and depends partially on the screening method used. Additionally, the review gives an insight in the risk factors most commonly associated with PIP and the drug groups most commonly involved in PIP. The results can contribute to outlining cross-border and country-specific action plans to reduce PIP in primary care as they represent an important opportunity to improve the prescribing quality in the primary care setting. There is a need for randomized controlled trials evaluating interventions that resolve PIP in the most cost-effective way to improve patient-related outcomes such as quality of life and to prevent drug-related problems leading to hospitalizations. A formal and straightforward screening method can be a great support in the evaluation of the patient’s pharmacotherapy, but should always be embedded in a global patient assessment by a multidisciplinary care team. Only then, positive effects on patients’ health outcomes can be shown [95]. Moreover, the complexity of the matter makes it unlikely that a single intervention at one point in the medication management process will be sufficient to tackle PIP. It had been demonstrated that multidisciplinary interventions including education and a systematic screening method are the most efficient for resolving and preventing all types of PIP [96–98].
References
Statistical Office of the European Communities. EUROSTAT: population data—proportion of population aged 65 and over. Secondary EUROSTAT: population data—proportion of population aged 65 and over. http://ec.europa.eu/eurostat/tgm/table.do?tab=table&init=1&language=en&pcode=tps00028&plugin=1
Al Hamid A, Ghaleb M, Aljadhey H et al (2014) A systematic review of hospitalization resulting from medicine-related problems in adult patients. Br J Clin Pharmacol 78(2):202–17
Tache SV, Soennichsen A, Ashcroft DM (2011) Prevalence of adverse drug events in ambulatory care: a systematic review. Ann Pharmacother 45(7-8):977–89
Thomsen LA, Winterstein AG, Sondergaard B et al (2007) Systematic review of the incidence and characteristics of preventable adverse drug events in ambulatory care. Ann Pharmacother 41(9):1411–26
Lund BC, Carnahan RM, Egge JA et al (2010) Inappropriate prescribing predicts adverse drug events in older adults. Ann Pharmacother 44(6):957–63
Hamilton H, Gallagher P, Ryan C et al (2011) Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med 171(11):1013–9
Price SD, Holman CD, Sanfilippo FM et al (2014) Association between potentially inappropriate medications from the Beers criteria and the risk of unplanned hospitalization in elderly patients. Ann Pharmacother 48(1):6–16
Pasina L, Djade CD, Tettamanti M et al (2014) Prevalence of potentially inappropriate medications and risk of adverse clinical outcome in a cohort of hospitalized elderly patients: results from the REPOSI Study. J Clin Pharm Ther 39(5):511–5
Laroche ML, Charmes JP, Nouaille Y et al (2007) Is inappropriate medication use a major cause of adverse drug reactions in the elderly? Br J Clin Pharmacol 63(2):177–86
Opondo D, Eslami S, Visscher S, et al. (2012) Inappropriateness of medication prescriptions to elderly patients in the primary care setting: a systematic review. PloS one 7(8)
Fick DM, Cooper JW, Wade WE et al (2003) Updating the Beers criteria for potentially inappropriate medication use in older adults—results of a US consensus panel of experts. Arch Intern Med 163(22):2716–24
Spinewine A, Schmader KE, Barber N et al (2007) Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet 370(9582):173–84
Hanlon JT, Schmader KE (2013) The medication appropriateness index at 20: where it started, where it has been, and where it may be going. Drugs Aging 30(11):893–900
(2012) American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. Journal of the American Geriatrics Society 60(4):616-3.
O'Mahony D, O'Sullivan D, Byrne S, et al. (2014) STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age and ageing
Holt S, Schmiedl S, Thurmann PA (2010) Potentially inappropriate medications in the elderly: the PRISCUS list. DtschArztebl Int 107(31-32):543–51
Hill-Taylor B, Sketris I, Hayden J et al (2013) Application of the STOPP/START criteria: a systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther 38(5):360–72
Shade MY, Berger AM, Chaperon C (2014) Potentially inappropriate medications in community-dwelling older adults. Res Gerontol Nurs 7(4):178–92
Gallagher PF, O'Connor MN, O'Mahony D (2011) Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther 89(6):845–54
Beers MH, Ouslander JG, Rollingher I et al (1991) Explicit criteria for determining inappropriate medication use in nursing home residents. UCLA Division of Geriatric Medicine. Arch Intern Med 151(9):1825–32
Aparasu RR, Mort JR (2000) Inappropriate prescribing for the elderly: Beers criteria-based review. Ann Pharmacother 34(3):338–46
Gallagher P, Barry P, O'Mahony D (2007) Inappropriate prescribing in the elderly. J Clin Pharm Ther 32(2):113–21
Galvin R, Moriarty F, Cousins G et al (2014) Prevalence of potentially inappropriate prescribing and prescribing omissions in older Irish adults: findings from The Irish LongituDinal Study on Ageing study (TILDA). Eur J Clin Pharmacol 70(5):599–606
Kovacevic SV, Simisic M, Rudinski SS, et al. (2014) Potentially inappropriate prescribing in older primary care patients. PloS one 9(4)
Primejdie D, Bojita M, Popa A (2012) Potential inappropriate medication use in community-dwelling elderly patients. A qualitative study. Farmacia 60(3):366–78
Blanco-Reina E, Ariza-Zafra G, Ocana-Riola R et al (2014) 2012 American Geriatrics Society Beers Criteria: enhanced applicability for detecting potentially inappropriate medications in European older adults? A comparison with the screening tool of older person’s potentially inappropriate prescriptions. J Am Geriatr Soc 62(7):1217–23
Shamseer L, Moher D, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Br Med J (Clin Res Ed) 349:g7647
WHO collaborating centre for drug statistics methodology. ATC/DDD Index 2015
Koper D, Kamenski G, Flamm M et al (2013) Frequency of medication errors in primary care patients with polypharmacy. Fam Pract 30(3):313–19
Vlahovic-Palcevski V, Bergman U (2004) Quality of prescribing for the elderly in Croatia-computerized pharmacy data can be used to screen for potentially inappropriate prescribing. Eur J Clin Pharmacol 60(3):217–20
Beers MH (1997) Explicit criteria for determining potentially inappropriate medication use by the elderly. An update. Arch Intern Med 157(14):1531–6
Popovic B, Quadranti NR, Matanovic SM et al (2014) Potentially inappropriate prescribing in elderly outpatients in Croatia. Eur J Clin Pharmacol 70(6):737–44
Mimica Matanovic S, Vlahovic-Palcevski V (2012) Potentially inappropriate medications in the elderly: a comprehensive protocol. Eur J Clin Pharmacol 68(8):1123–38
Bregnhoj L, Thirstrup S, Kristensen MB et al (2007) Prevalence of inappropriate prescribing in primary care. Pharm World Sci 29(3):109–15
Hanlon JT, Schmader KE, Samsa GP et al (1992) A method for assessing drug therapy appropriateness. J Clin Epidemiol 45(10):1045–51
Pitkala KH, Strandberg TE, Tilvis RS (2002) Inappropriate drug prescribing in home-dwelling, elderly patients: a population-based survey. Arch Intern Med 162(15):1707–12
Leikola S, Dimitrow M, Lyles A et al (2011) Potentially inappropriate medication use among Finnish non-institutionalized people aged ≥65 years. A register-based, cross-sectional, national study. Drugs Aging 28(3):227–36
Bell JS, Ahonen J, Lavikainen P et al (2013) Potentially inappropriate drug use among older persons in Finland: application of a new national categorization. Eur J Clin Pharmacol 69(3):657–64
Finnish Medicines Agency. Database of medication for the elderly. Secondary database of medication for the elderly 2010. http://www.fimea.fi/development/medicines_information/database_of_medication_for_the_elderly
Lechevallier-Michel N, Gautier-Bertrand M, Alperovitch A et al (2005) Frequency and risk factors of potentially inappropriate medication use in a community-dwelling elderly population: results from the 3C Study. Eur J Clin Pharmacol 60(11):813–9
Berdot S, Bertrand M, Dartigues JF et al (2009) Inappropriate medication use and risk of falls—a prospective study in a large community-dwelling elderly cohort. BMC Geriatr 9:30
Laroche ML, Charmes JP, Merle L (2007) Potentially inappropriate medications in the elderly: a French consensus panel list. Eur J Clin Pharmacol 63(8):725–31
Bongue B, Naudin F, Laroche ML et al (2009) Trends of the potentially inappropriate medication consumption over 10 years in older adults in the East of France. Pharmacoepidemiol Drug Saf 18(12):1125–33
Bongue B, Laroche ML, Gutton S et al (2011) Potentially inappropriate drug prescription in the elderly in France: a population-based study from the French National Insurance Healthcare system. Eur J Clin Pharmacol 67(12):1291–9
Jardin M, Bocquier A, Cortaredona S et al (2012) Potentially inappropriate prescriptions for the elderly: a study of health insurance reimbursements in Southeastern France. Rev Epidemiol Sante Publique 60(2):121–30
Fiss T, Dreier A, Meinke C et al (2011) Frequency of inappropriate drugs in primary care: analysis of a sample of immobile patients who received periodic home visits. Age Ageing 40(1):66–73
Amann U, Schmedt N, Garbe E (2012) Prescribing of potentially inappropriate medications for the elderly: an analysis based on the PRISCUS list. Dtsch Arztebl Int 109(5):69–75
Goltz L, Kullak-Ublick GA, Kirch W (2012) Potentially inappropriate prescribing for elderly outpatients in Germany: a retrospective claims data analysis. Int J Clin Pharmacol Ther 50(3):185–94
Schubert I, Kupper-Nybelen J, Ihle P et al (2013) Prescribing potentially inappropriate medication (PIM) in Germany’s elderly as indicated by the PRISCUS list. An analysis based on regional claims data. Pharmacoepidemiol Drug Saf 22(7):719–27
Zimmermann T, Kaduszkiewicz H, van den Bussche H et al (2013) Potentially inappropriate medication in elderly primary care patients. A retrospective, longitudinal analysis. Bundesgesundheitsbl-Gesundheitsforsch-Gesundheitsschutz 56(7):941–49
Linder R, Schneider U, Kothemann M et al (2014) [Physicians’ prescription behavior of potentially inappropriate medications for elderly people: an analysis using the PRISCUS list based on TK routine data]. Wochenschr (1946) 139(19):983–9
Ryan C, O'Mahony D, Kennedy J et al (2009) Appropriate prescribing in the elderly: an investigation of two screening tools, Beers criteria considering diagnosis and independent of diagnosis and improved prescribing in the elderly tool to identify inappropriate use of medicines in the elderly in primary care in Ireland. J Clin Pharm Ther 34(4):369–76
McLeod PJ, Huang AR, Tamblyn RM et al (1997) Defining inappropriate practices in prescribing for elderly people: a national consensus panel. CMAJ Can Med Assoc J J de l'Assoc Med Can 156(3):385–91
Ryan C, O'Mahony D, Kennedy J et al (2009) Potentially inappropriate prescribing in an Irish elderly population in primary care. Br J Clin Pharmacol 68(6):936–47
Ryan C, O'Mahony D, Kennedy J et al (2008) Screening tools for elderly patients in primary care: STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions) and START (Screening Tool to Alert doctors to Right, i.e., appropriate, indicated Treatment). Pharmacoepidemiol Drug Saf 17(7):746–47
Cahir C, Fahey T, Teeling M et al (2010) Potentially inappropriate prescribing and cost outcomes for older people: a national population study. Br J Clin Pharmacol 69(5):543–52
Cahir C, Bennett K, Teljeur C et al (2014) Potentially inappropriate prescribing and adverse health outcomes in community dwelling older patients. Br J Clin Pharmacol 77(1):201–10
Maio V, Yuen EJ, Novielli K et al (2006) Potentially inappropriate medication prescribing for elderly outpatients in Emilia Romagna, Italy: a population-based cohort study. Drugs Aging 23(11):915–24
Landi F, Russo A, Liperoti R et al (2007) Impact of inappropriate drug use on physical performance among a frail elderly population living in the community. Eur J Clin Pharmacol 63(8):791–9
Lapi F, Pozzi C, Mazzaglia G et al (2009) Epidemiology of suboptimal prescribing in older, community dwellers: a two-wave, population-based survey in Dicomano, Italy. Drugs Aging 26(12):1029–38
Maio V, Del Canale S, Abouzaid S (2010) Using explicit criteria to evaluate the quality of prescribing in elderly Italian outpatients: a cohort study. J Clin Pharm Ther 35(2):219–29
Bradley MC, Fahey T, Cahir C et al (2012) Potentially inappropriate prescribing and cost outcomes for older people: a cross-sectional study using the Northern Ireland Enhanced Prescribing Database. Eur J Clin Pharmacol 68(10):1425–33
Nyborg G, Straand J, Brekke M (2012) Inappropriate prescribing for the elderly—a modern epidemic? Eur J Clin Pharmacol 68(7):1085–94
Rognstad S, Brekke M, Fetveit A et al (2009) The Norwegian General Practice (NORGEP) criteria for assessing potentially inappropriate prescriptions to elderly patients. Scand J Prim Health Care 27(3):153–59
Rajska-Neumann A, Wieczorowska-Tobis K (2007) Polypharmacy and potential inappropriateness of pharmaco-logical treatment among community-dwelling elderly patients. Arch Gerontol Geriatr 44(Suppl 1):303–9
de Oliveira MS, Soares MA, Foppe van Mil JW et al (2006) Inappropriate drug use by Portuguese elderly outpatients—effect of the Beers criteria update. Pharm World Sci PWS 28(5):296–301
Barnett K, McCowan C, Evans JMM et al (2011) Prevalence and outcomes of use of potentially inappropriate medicines in older people: cohort study stratified by residence in nursing home or in the community. BMJ Qual Saf 20(3):275–81
Gavilan Moral E, Morales Suarez-Varela MT, Hoyos Esteban JA et al (2006) Inappropriate multiple medication and prescribing of drugs immobile elderly patients living in the community. Aten Primaria / Soc Esp de Med de Familia Comunitaria 38(9):476–80
Conejos Miguel MD, Sanchez Cuervo M, Delgado Silveira E et al (2010) Potentially inappropriate drug prescription in older subjects across health care settings. Eur Geriatr Med 1(1):9–14
Mera F, Mestre D, Almeda J et al (2011) Inappropriate prescription in the community elderly, are we aware of? Revi Esp Geriatr Gerontol 46(3):125–30
Lesende IM, Crespo IM, Lopez GM et al (2013) Potentiality of STOPP/START criteria used in primary care to effectively change inappropriate prescribing in elderly patients. Eur Geriatr Med 4(5):293–98
Parodi Lopez N, Villan Villan YF, Granados Menendez MI et al (2014) Potentially inappropriate prescribing in patients over 65 years old in a primary care health centre. Aten Primaria 46(6):290–97
Klarin I, Wimo A, Fastbom J (2005) The association of inappropriate drug use with hospitalisation and mortality: a population-based study of the very old. Drugs Aging 22(1):69–82
Robertson HA, MacKinnon NJ (2002) Development of a list of consensus-approved clinical indicators of preventable drug-related morbidity in older adults. Clin Ther 24(10):1595–613
Johnell K, Fastbom J, Rosen M et al (2007) Inappropriate drug use in the elderly: a nationwide register-based study. Ann Pharmacother 41(7):1243–8
National Board of Health and Welfare (Socialstyrelsen). Indikatorer för god läkemedelsterapi hos äldre Secondary Indikatorer för god läkemedelsterapi hos äldre 2003. http://www.socialstyrelsen.se/publikationer2010/2010-6-29
Blozik E, Rapold R, von Overbeck J et al (2013) Polypharmacy and potentially inappropriate medication in the adult, community-dwelling population in Switzerland. Drugs Aging 30(7):561–8
Reich O, Rosemann T, Rapold R et al (2014) Potentially inappropriate medication use in older patients in Swiss managed care plans: prevalence, determinants and association with hospitalization. PLoS One 9(8):e105425
van der Hooft CS, Jong GW t, Dieleman JP et al (2005) Inappropriate drug prescribing in older adults: the updated 2002 Beers criteria—a population-based cohort study. Br J Clin Pharmacol 60(2):137–44
Denneboom W, Dautzenberg MGH, Grol R et al (2006) Analysis of polypharmacy in older patients in primary care using a multidisciplinary expert panel. Br J Gen Pract 56(528):504–10
Ay P, Akici A, Harmanc H (2005) Drug utilization and potentially inappropriate drug use in elderly residents of a community in Istanbul, Turkey. Int J Clin Pharmacol Ther 43(4):195–202
Yayla ME, Bilge U, Binen E, et al. (2013) The use of START/STOPP criteria for elderly patients in primary care. Scientific World Journal
De Wilde S, Carey IM, Harris T et al (2007) Trends in potentially inappropriate prescribing amongst older UK primary care patients. Pharmacoepidemiol Drug Saf 16(6):658–67
Carey IM, De Wilde S, Harris T et al (2008) What factors predict potentially inappropriate primary care prescribing in older people? Analysis of UK primary care patient record database. Drugs Aging 25(8):693–706
Bradley MC, Motterlini N, Padmanabhan S, et al. (2014) Potentially inappropriate prescribing among older people in the United Kingdom. BMC geriatrics 14
Fialova D, Onder G (2009) Medication errors in elderly people: contributing factors and future perspectives. Br J Clin Pharmacol 67(6):641–45
Guaraldo L, Cano FG, Damasceno GS et al (2011) Inappropriate medication use among the elderly: a systematic review of administrative databases. BMC Geriatr 11:79
Tommelein E, Petrovic M, Somers A, et al. (2015) Older patients’ prescriptions screening in the community pharmacy: development of the Ghent Older People’s Prescriptions community pharmacy screening (GheOP(3)S) tool. J Public Health (Oxf)
Bulajeva A, Labberton L, Leikola S et al (2014) Medication review practices in European countries. Res Soc Adm Pharm RSAP 10(5):731–40
Zhang Y, Baicker K, Newhouse JP (2010) Geographic variation in the quality of prescribing. N Engl J Med 363(21):1985–8
O'Mahony D, O'Sullivan D, Byrne S et al (2015) STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 44(2):213–8
Lindblad CI, Hanlon JT, Gross CR et al (2006) Clinically important drug-disease interactions and their prevalence in older adults. Clin Ther 28(8):1133–43
Pugh MJV, Starner CI, Amuan ME et al (2011) Exposure to potentially harmful drug-disease interactions in older community-dwelling veterans based on the healthcare effectiveness data and information set quality measure: who is at risk? J Am Geriatr Soc 59(9):1673–78
Aspinall SL, Zhao X, Semla TP et al (2015) Epidemiology of drug-disease interactions in older veteran nursing home residents. J Am Geriatr Soc 63(1):77–84
Onder G, van der Cammen TJ, Petrovic M et al (2013) Strategies to reduce the risk of iatrogenic illness in complex older adults. Age Ageing 42(3):284–91
Kaur S, Mitchell G, Vitetta L et al (2009) Interventions that can reduce inappropriate prescribing in the elderly. A systematic review. Drugs Aging 26(12):1013–28
Meid AD, Lampert A, Burnett A, et al. (2015) The impact of pharmaceutical care interventions for medication underuse in older people: a systematic review and meta-analysis. British journal of clinical pharmacology
Cullinan S, Fleming A, O'Mahony D et al (2015) Doctors’ perspectives on the barriers to appropriate prescribing in older hospitalized patients: a qualitative study. Br J Clin Pharmacol 79(5):860–9
Funding
None
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Tommelein, E., Mehuys, E., Petrovic, M. et al. Potentially inappropriate prescribing in community-dwelling older people across Europe: a systematic literature review. Eur J Clin Pharmacol 71, 1415–1427 (2015). https://doi.org/10.1007/s00228-015-1954-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-015-1954-4