Abstract
Background:
Human papillomavirus (HPV) vaccination of girls will have relatively little effect on HPV-related disease in men who have sex with men (MSM). We determined HPV prevalence and risk factors in MSM to inform the potential effectiveness of vaccinating MSM.
Methods:
Cross-sectional study of 522 MSM aged 18–40 attending a London sexual health clinic who completed a computer-assisted self-interview. Urine and two swabs (anal and penile/scrotal/perianal) were collected and tested using an in-house Luminex-based HPV genotyping system.
Results:
Prevalence of DNA of the vaccine-preventable HPV types in ano-genital specimens of men was 87/511 (17.0%), 166/511 (32.5%) and 232/511 (45.4%) for the bivalent (HPV16/18), quadrivalent (HPV6/11/16/18) and nonavalent (HPV6/11/16/18/31/33/45/52/58) vaccine types, respectively. A total of 25.1% had one of the quadrivalent types, and 7.4% had 2+ types. Median age at first anal sex was 19 (IQR 17–23) and at first clinic attendance was 24 (IQR 20–27). The increase in the odds of any HPV infection per year of age was 4.7% (95% CI 1.2–8.4).
Conclusions:
On the basis of the current infection status, most MSM, even among a high-risk population attending a sexual health clinic, are not currently infected with the vaccine-type HPV. A targeted vaccination strategy for MSM in the UK could have substantial benefits.
Similar content being viewed by others
Main
Human papillomavirus (HPV) types 16/18 are common sexually transmitted viruses that are classified as high-risk (HR-HPV) because persistent infections are associated with ano-genital and other cancers including the head and neck (Bouvard et al, 2009). Two vaccines against HPV16/18 infection are now widely used. The bivalent vaccine (Cervarix) protects against HPV16/18. The quadrivalent vaccine (Gardasil) protects against HPV16/18 and the low-risk types HPV6/11 which are responsible for the majority of genital warts (Schiller et al, 2012). A nonavalent vaccine targeting five additional HR-HPV types, 31/33/45/52/58 (Bouvard et al, 2009), responsible for a further 20% of cervical cancers (Muñoz et al, 2003; De Sanjose et al, 2010), is expected to be licensed (Joura et al, 2014; Olsson et al, 2014). In 2008, the UK introduced an HPV immunisation programme for adolescent girls, primarily as protection from cervical cancer (Jit et al, 2008; Department of Health, United Kingdom Government, 2014). With over 80% coverage among young girls, herd immunity is expected to reduce HPV infection and associated disease in unvaccinated females and heterosexual males, as has been observed in Australia and Sweden (Leval et al, 2012; Ali et al, 2013). Provided vaccine coverage was high, including boys in the UK programme was considered not cost-effective, but this is being reconsidered (Jit et al, 2008). Men who have sex with men (MSM) are unlikely to benefit from herd immunity with the current programme (Ali et al, 2013), are more likely to be infected with HPV (Nyitray et al, 2011) and have relatively high rates of HPV-related disease, particularly genital warts (Anic et al, 2012) and anal cancer (Frisch et al, 2003). Anal cancer incidence is increasing and is estimated at 1, 5 and 46/100 000 person years in all men, HIV-negative MSM and HIV-positive MSM, respectively (Frisch et al, 2003; Wilkinson et al, 2014).
Quadrivalent vaccine trials show efficacy against anal intraepithelial neoplasia (AIN) in men. In participants with no evidence of prior infection receiving three doses, vaccination was 78% (95% CI 40–93) efficacious against HPV 6/11/16/18-related AIN and 95% (95% CI 80–99) against persistent anal HPV infection (6/11/16/18) in 16–26 year old MSM (Palefsky et al, 2011), and 90% (95% CI 69–98) efficacious against 6/11/16/18-related external genital lesions (principally genital warts; Giuliano et al, 2011b). In the intention-to-treat analyses, including those with prior HPV infection, efficacy was lower, but still substantial, at 50% (95% CI 26–67) against AIN and 66% (95% CI 41–74) against genital warts. Given the efficacy against cervical, vulval, vaginal and now anal disease, it is expected that vaccines will protect against other HPV16/18-related cancers for example penile and oropharyngeal. Vaccination may also benefit previously exposed individuals (Swedish et al, 2012; Swedish and Goldstone, 2014).
Prevalence of HPV DNA in genital specimens does not measure cumulative exposure as 70% of genital high-risk HPV (HR-HPV) infections in men clear by 12 months (Giuliano et al, 2011a). Nevertheless, age-specific DNA prevalence, linked to behavioural data, gives valuable insights into the epidemiology of HPV. A systematic review of HPV in MSM reported a pooled anal prevalence estimate of 64% for any HPV and 37% for any HR-HPV in HIV-negative MSM (Machalek et al, 2012).
Given the relatively high rates of HPV-related ano-genital disease, lack of herd immunity, and the increasing recognition of the burden of other HPV-related disease, the question arises: is it worthwhile to vaccinate MSM? Sexual health clinics (SHC) may offer a convenient setting to vaccinate MSM. In the absence of evidence of any therapeutic benefits (in clearing or preventing disease owing to prevalent HPV infections; The FUTURE II Study Group, 2007), the expected benefit of HPV vaccination in this setting is maximised when the prior exposure level is low but the future risk of acquiring vaccine-preventable infections and disease remains high.
No studies in the UK have estimated the prevalence of the four HPV types targeted by the quadrivalent vaccine in anal canal samples in HIV-negative MSM (Sayers et al, 1998; Lacey et al, 1999). Five studies (from Peru (Quinn et al, 2012), Italy (Donà et al, 2012), Thailand (Phanuphak et al, 2013) and the Netherlands (Van der Snoek et al, 2005; Van Aar et al, 2013) with a sample size >100 persons have estimated the prevalence of the four HPV types 6/11/16/18 in the anal canal of HIV-negative MSM recruited in SHCs, with one also measuring penile HPV DNA (Van Aar et al, 2013).
The Mortimer Market Centre (MMC), in London, is one of the largest outpatient SHCs in Europe with ∼80 000 patient attendances per year (∼25% are MSM). We conducted a study to estimate the age- and type-specific prevalence of HPV DNA, including quadrivalent (HPV6/11/16/18) and nonavalent vaccine types (HPV6/11/16/18/31/33/45/52/58). We determined the demographic, behavioural and health service use characteristics associated with quadrivalent types, among MSM attending this clinic to examine the potential benefit, barriers and opportunities for vaccinating this group in this setting, whether with the bivalent, quadrivalent or nonavalent vaccines.
Methods
Recruitment and study procedures
Men attending the MMC, who reported anal or oral sex with another man in the last five years, aged 16–40 years inclusive, were consented to participate in this cross-sectional study. With an estimate for HPV prevalence of 16%, a sample size of 530 men would provide 80% power to detect a difference between two equally sized subgroups (e.g., age groups) at the 5% significance level if the true prevalence in the subgroups is 12% and 21%, that is, a relative risk of 1.75.
Participants completed a computer-assisted self-interview (CASI) questionnaire covering demographics, sexual behaviour, history of sexually transmitted infections (including HIV), knowledge of HPV, attitudes to HPV vaccination and health service use. Specimens were collected as follows: an anal specimen was obtained by inserting a sterile plastic flocked swab that was pre-soaked in sterile saline 3 cm into the anal canal and rotated 360 degrees; an external genital specimen was obtained by rubbing a similar swab on the glans penis/coronal sulcus, penile shaft including the prepuce (if present), scrotum, and perianal area; a first-void urine specimen was collected into a 30 ml universal collection tube. Swabs were placed in UTM-RT medium (Copan Diagnostics, Murrieta, CA, USA). Specimens were stored immediately at 4 °C and transferred in cold condition to the laboratory within 24 h.
STI diagnoses within 30 days of the study visit and HIV test history were recorded (Health protection Agency, 2014).
Laboratory methods
In the laboratory, swab specimens were vortexed to agitate the material from the swab into the buffer and aliquots of 300 μl were stored at −80 °C. Urine specimens (1 ml) were centrifuged (13 000 r.p.m. for 20 min) and the pellet was resuspended in 300 μl sterile phosphate-buffered saline prior to storage at −80 °C. Specimens were removed for batch processing wherein all available specimens from each individual were processed for DNA extraction and HPV testing in the same run. Following lysis with 40 μl Qiagen Protease and 265 μl Qiagen buffer, nucleic acid was extracted on a BioRobot Universal platform using QIAampDNA Blood BioRobot MDx kit (Qiagen, Crawley, West Sussex, UK). Ten microlitres of the 100 μl elution were used for PCR amplification using the in-house single-round multiplex PCR and type-specific infections were resolved using a genotyping assay based on the Bio-Plex (Luminex xMAP, Bio-Rad Laboratories, Hemel Hempstead, Hertfordshire, UK) platform (Bissett et al, 2011). Specimen integrity was established by incorporating a control PCR targeting the human pyruvate-dehydrogenase gene. HPV genotypes 16/18/31/33/35/39/45/51/52/56/58/59/68 were considered as HR-HPV types, HPV genotypes 6/11 were considered as low-risk HPV (LR-HPV) types whereas HPV genotypes 26/53/66/70/73/82 were considered as possible HR-HPV (Bouvard et al, 2009).
Statistical methods
Human papillomavirus DNA prevalence was defined as the proportion of participants with detectable HPV DNA in ⩾1 of the three specimen types in participants with ⩾1 specimen type that was adequate for PCR. We estimated HPV DNA prevalence by HPV type, HIV status, specimen type and age. Confidence intervals (95%) were determined by the Clopper–Pearson (exact) method. Human immunodeficiency virus status was determined from both questionnaire data (self-reported) and clinical records at MMC.
We used univariate logistic regression to explore the age-relationship with HPV infection (any type, HPV16/18, any quadrivalent vaccine types, any nonavalent vaccine types, any HR-HPV, and HPV6/11). To explore the potential for future HPV acquisition at any age, we examined the effect of age on sexual behaviour using linear regression. Demographic and behavioural risk factors for quadrivalent vaccine types were assessed using logistic regression and we present age-adjusted odds ratios (aOR) with 95% confidence intervals. Health service use, HPV knowledge and STI risk perception, by quadrivalent HPV type status, were explored to identify potential opportunities and barriers for delivery of vaccination. Statistical analyses were conducted using STATA v13.1 (StataCorp LP, College Station, TX, USA).
Ethical review
The study was reviewed by the Camden and Islington Research ethics committee (REC reference number: 09/H0722/71) and received NHS approval (R&D ref: CSP 30296).
Results
Participation and response
A total of 522 MSM were recruited between October 2010 and July 2012. The participation rate was estimated at 81.4% from the enrolment log. All analyses were restricted to the 511 participants (97.9%) that had ⩾1 specimen type that was valid for PCR. Of these, 495 completed a CASI form.
Participant characteristics
Table 1 shows demographic and behavioural characteristics of study participants. The median age was 30 years (IQR 25–35), with 17 participants (3.3%) younger than 21 years. Nearly half (46.9%) were born in the UK and another 38.4% had lived in the UK for at least 3 years. The majority (76.2%) were of white ethnicity. Overall 91.5% self-identified as gay/homosexual. Most participants were employed (78.8%), with ⩾3 years of education post-16 (68.7%). Two-thirds (65.9%) reported higher risk drinking behaviour (identified using the alcohol use and disorders identification test; AUDIT-C) and one-third (29.4%) were current smokers. A total of 28.9% were circumcised and 27 participants (5.3%) had been diagnosed as HIV-positive and 28 (5.7%) had never had an HIV test.
The study population broadly mirrors that of MSM attending MMC in terms of age and ethnicity, but were slightly younger (data not shown). Men who have sex with men attending other SHCs in England are younger and less ethnically diverse (Public Health England, personal communication).
Human papillomavirus prevalence
The prevalence of HPV at any site was 72.2% (95% CI 68.2–75.9) (Table 2). The prevalence of HR-HPV types was 47.2% (95% CI 42.9–51.5). The most common HPV types were 16, 11 and 6 with prevalence estimates of 13.5% (95% CI 10.8–16.8), 11.5% (95% CI 9.0–14.6) and 9.4% (95% CI 7.1–12.3), respectively. At least one quadrivalent vaccine type was detected in 32.5% of participants; 18.4% (95% CI 15.3–22.0) had a bivalent type(s); 18.6% (95% CI 15.4–22.2) had types 6 and/or 11. The 27 HIV-positive MSM had a higher HPV prevalence (92.6%; 95% CI 75.7–99.1) compared with the 484 HIV-negative MSM (71.1%; 95% CI 66.8–75.1). Similarly, HIV-positive MSM had higher prevalence of quadrivalent (59.3% (38.8–77.6) vs 31.0% (26.9–35.3)) and nonavalent (74.1% (53.7–88.9) vs 43.8% (39.3–48.4)) vaccine types.
Table 3 shows the prevalence of vaccine-preventable HPV types by specimen type. A total of 132 men (29.1%) had at least one detectable quadrivalent type in the anal canal. A further 28 men had quadrivalent types detected in the external genital swab so that the prevalence in these two samples was 32.1%. Although only 16 men (3.3%) had detectable quadrivalent types in their urine, 6 of these infections were in addition to the 160 detected in the two swabs.
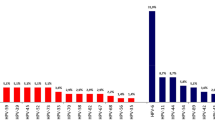
Figure 1 shows age-specific prevalence of HPV. For any HPV type, and modelling age as a continuous variable (18–40 years), there was a 4.7% (95% CI 1.2–8.4) increase in the odds of an HPV infection per year. The effect was not significant when examining age as a categorical variable, but confidence intervals are wide, and there were few participants in the youngest category in particular (18–20 years). There was no significant association between age and HR-HPV, bivalent, quadrivalent or nonavalent vaccine-type detection, considered separately. There was a 2.5% increase in the odds of acquiring a HR-HPV infection per year between the ages of 18 and 40, although this was not statistically significant (P=0.12). The increase was 7.0% per year (95% CI −0.2–17.3) from 21 to 30 years (P=0.14) and then decreased by 2.3% per year between 31 and 40 (P=0.61).
Age-specific prevalence of HPV DNA at any site in MSM. Data available in Supplementary Table 2.
Sexual behaviour
The majority of men (51.2%) reported first receptive anal sex between 16–20 years old (Table 1). The median age at first oral, insertive anal and receptive anal sex with a man was 18 (IQR 16–20), 19 (IQR 17–22) and 19 (IQR 17–23), respectively. Two-thirds of men (66.2%) had had >30 lifetime male-sex (anal or oral) partners and only six men (1.2%) had fewer than three lifetime partners. Of those who had an anal sex partner in the last year, 62.6% had sex with at least one partner without a condom. For each year increase in age, the odds of having >30 lifetime partners increased by 14.1% (95% CI 10.0–18.4) but there was no association between age and the number of partners, new partners or partners without a condom in the last year. For example, the median number of male anal partners in the last year was 6 (IQR 3–15) in men aged 18–30 and 8 (IQR 3–20) in men aged 31–40. Younger men, aged 18–30, were more likely to have had receptive anal sex in the last year than older men (86% vs 77%; OR 1.8; 95% CI 1.1–2.9). The proportion of men who had sex without a condom in the last year was higher among younger men, (66% vs 58%; OR 1.4 95% CI 0.9–2.0) but this was not statistically significant.
Participants had more lifetime partners than MSM who had attended a SHC (in the last five years) in the British population in 2010–2012 measured in the Natsal-3 population-based survey. Within Natsal-3, MSM who had attended a SHC had more lifetime partners than those who had not. For example, 93% of MSM in our study reported >10 lifetime partners, compared with the 78% of MSM who had attended a SHC in Natsal-3 and with the 25% of MSM who had not attended. (Natsal-3 team, personal communication)
Risk factors for quadrivalent vaccine-type infection
Detection of any quadrivalent vaccine types was associated with number of lifetime anal or oral sex partners (aOR 2.6; 95% CI 1.4–4.8; in those with >100 compared with ⩽20 partners), an HIV-positive diagnosis (aOR 3.2; 95% CI 1.5–7.1) and rectal drug use (aOR 2.0; 95% CI 1.1–3.6) (Supplementary Table 1). There were no other statistically significant associations between quadrivalent type infection and demographic, health or behavioural variables, after adjusting for age.
Health-seeking behaviour, HPV knowledge and risk perception
The median age at first attending a SHC in the United Kingdom was 24 (IQR 20–27) (Table 4); 77% had visited their GP in the last year but only 44% of these had disclosed their sexuality; 88% had received at least one dose of hepatitis-B vaccine of whom 47% had received ⩾3 doses. Only 4% of the MSM reported that they would probably or definitely refuse the HPV vaccine if it was offered. There were no significant differences in health-seeking behaviour, HPV knowledge, STI risk perception or expected vaccine acceptance between those who had quadrivalent vaccine types detected and those who did not.
Discussion
This study, in over 500 MSM attending a London SHC, found that two-thirds (67.5%) of men did not have any of the quadrivalent vaccine-types detectable. A quarter (25.1%) had one type, 7.4% had two or three types and none had all four quadrivalent types detected. Our results are similar to those reported in a recent systematic review of anal canal HPV prevalence in MSM (Machalek et al, 2012). On the basis of the evidence of current infection, there is a potential benefit in vaccinating this group of men, which would be increased if the vaccine is also effective in those with previous exposure (Swedish et al, 2012; Swedish and Goldstone, 2014). These data can inform decisions on vaccination strategies for MSM, and mathematical models of effectiveness and cost-effectiveness.
The results suggest that extending from quadrivalent vaccine to the nonavalent vaccine could protect a further 13–22% of participants from HR-HPV infection. It is not clear how this translates into potentially preventable HPV-related disease. In men, it is estimated that quadrivalent vaccine could prevent up to 76% of anal cancers and 88% in women (Hoots et al, 2009). A further 7% of male anal cancers might be prevented with the nonavalent vaccine (Hoots et al, 2009).
As the MSM in our study are, on average, at higher risk, in terms of STI acquisition, than MSM generally in the United Kingdom, we will have underestimated the proportion of MSM who may benefit from vaccination.
Vaccine effectiveness is highest in HPV-naive individuals (Palefsky et al, 2011). Human papillomavirus DNA testing is a marker of current infection and gives no information on previous resolved episodes. The majority of HPV infections are transient, and the median time to clearance of genital HR-HPV is estimated to be 7.2 months (Giuliano et al, 2011a). Detecting HPV antibodies in serum may provide evidence of previous exposure, but lacks sensitivity owing to low seroconversion rates (Carter et al, 2000; Edelstein et al, 2011; Giuliano, 2014).
Men who have sex with men in our study reported attending a SHC for the first time at the age of 24, an average of five years after the initiation of anal sex. One in three MSM aged 18–23 years had detectable quadrivalent vaccine-type DNA. We estimate that, if 33% of MSM are infected at an age of 19 years (and those negative are all naive to the quadrivalent types), and prevalence remains at 33%, all men will have been infected by the age of 24.5 years with an incidence of 0.9%/month, if the average duration of infection is seven months and there is no natural immunity. Human immunodeficiency virus-negative MSM in Thailand had a 12-month cumulative incidence of 7.2% (95% CI 3.0–17.5%) and 33.9% (21.1–54.5%) for HPV16 and HR-HPV, respectively (Phanuphak et al, 2013). Thus it is unlikely that MSM will be completely HPV-naive for vaccine-preventable types by the time they first attend a SHC. That said, vaccine efficacy in MSM who are not HPV-naive has been demonstrated. In older MSM (⩾26 years), including MSM with a history of HPV infection, the quadrivalent vaccine was 55% (95% CI 8–78%) efficacious against anal warts after five years (Swedish and Goldstone, 2014); and in MSM (mean age 40), with a history of biopsy-confirmed and treated high-grade AIN (HGAIN; a precursor to anal cancer), it was 50% (95% CI 2–74%) efficacious against recurrent HGAIN after 2 years (Swedish et al, 2012). Furthermore, cost-effectiveness, taking into account indirect benefits, is not reliant on HPV-naivety in the whole population (Kim, 2010; Zou et al, 2014).
In our MSM population, aged 18–40 years, rates of partner change and prevalence of HPV did not decline with age and there was no association between detectable quadrivalent vaccine types and age at first sex. We found that the prevalence of any HPV infection increased with age but no significant associations were detectable when individual or grouped LR-HPV or HR-HPV were considered. The MSM in this study reported high levels of sexual risk behaviour. Therefore the risk of future infection with HPV is likely to be high across all age groups as demonstrated by comparing HPV prevalence in MSM populations with different age distributions (Zou et al, 2014). The rapidly diminishing benefit of vaccination with age seen in women, as a result of the age-association with cervical HPV infection, may not apply to MSM.
Our high estimates for ano-genital HPV prevalence in HIV-positive MSM are similar to those in larger European HIV-positive MSM populations (Parisi et al, 2011; Videla et al, 2013). Human immunodeficiency virus-positive MSM have more preventable HPV infection, and HPV vaccines have been shown to be safe and highly immunogenic in HIV-positive populations (Toft et al, 2014), so vaccination of HIV-positive MSM has great potential and cost-effectiveness analyses should stratify by HIV status.
Although the number in each age stratum was limited, our data suggest that HR-HPV prevalence increases until the approximate age of 30 years. This is consistent with the results from the largest HPV study (EXPLORE), in HIV-negative MSM in the United States of America (n=1409; median age 37; IQR 31–43), which found no overall association with age (any type, LR-HPV or HR-HPV) but did show an increase in HR-HPV through the age-bands from <25 years until 30–34 years (Chin-Hong et al, 2004). After the age of 30 years, HR-HPV prevalence may slowly decline with age as was shown in the EXPLORE study (Chin-Hong et al, 2004) and the HIM study (for both any HPV and HR-HPV (Nyitray et al, 2011)). We found a non-significant decrease of HR-HPV with age between 31 and 40 years. The lack of age-association across the entire age range in EXPLORE could be because 78% were 30 years or older (Chin-Hong et al, 2004).
This is the first study to estimate the prevalence of all HPV genotypes included in the bivalent, quadrivalent and nonavalent vaccines, with sampling at multiple genital sites, in a predominantly HIV-negative population of MSM attending a SHC. It is the first large study of HPV prevalence in MSM in the United Kingdom, recruited from a large SHC in London. This study has some limitations including that not all men were tested for HIV at the clinic visit so there is a potential for undiagnosed HIV. This potential misclassification would overestimate the HPV prevalence in HIV-negative MSM. The sensitivity for the detection of HPV DNA in urine is lower than genital swabs (Pathak et al, 2014) and we observed a low HPV prevalence in this specimen type. Despite low prevalence, we did identify HPV DNA in the urine of some participants when it had not been detected in other specimens, differences in HPV test sensitivity/specificity and specimen quality across specimen types may contribute to non-concordance.
Sexual health clinics provide an opportunity to vaccinate MSM, a group at high risk of HPV infection, who do not readily identify themselves to GPs. Forty-five percent of MSM in a national survey in Britain between 2010–2012 had attended a SHC in the last five years (Sonnenberg et al, 2013). The HPV prevalence and sexual activity levels that we report show that vaccinating MSM attending SHCs who have the highest STI transmission risk behaviours would undoubtedly incur a lower vaccine efficacy than vaccinating all MSM (however that might be achieved) or all adolescent males. However, the indirect benefits for other MSM, who are not attending SHCs, may be substantial given the current high infection rates, and help to compensate for the reduction in efficacy. Even if a country such as the United Kingdom were to include all adolescent boys in a routine vaccination programme, there would be potential benefit in vaccinating MSM for some years as a catch-up programme.
In this study of MSM with high numbers of lifetime partners, no participant had all four vaccine-preventable HPV types detected in their genital specimens. Many are likely to have encountered and resolved infections prior to the study. As vaccine effectiveness has been demonstrated in older HPV-exposed MSM, and there appears to be a continuing risk of acquiring new HPV infections, there is potential benefit in vaccinating this group, at least with the quadrivalent vaccine. The nonavalent vaccine would provide additional protection, but the benefit in disease prevention would need to be assessed further. The results reported here are necessary to inform models that quantify the impact and cost-effectiveness of HPV vaccination strategies.
Change history
28 April 2015
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Van Aar F, Mooij SH, van der Sande MAB, AGCL S, Stolte IG, M CJLM, Verhagen DWM, King AJ, de Vries HJC, Schim van der Loeff MF (2013) Anal and penile high-risk human papillomavirus prevalence in HIV-negative and HIV-infected MSM. AIDS 27: 2921–2931.
Ali H, Donovan B, Wand H, Read TRH, Regan DG, Grulich AE, Fairley CK, Guy RJ (2013) Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data. BMJ 346: f2032.
Anic GM, Lee J-H, Villa LL, Lazcano-Ponce E, Gage C, José C, Silva R, Baggio ML, Quiterio M, Salmerón J, Papenfuss MR, Abrahamsen M, Stockwell H, Rollison DE, Wu Y, Giuliano AR (2012) Risk factors for incident condyloma in a multinational cohort of men: the HIM study. J Infect Dis 205: 789–793.
Bissett SL, Howell-Jones R, Swift C, De Silva N, Biscornet L, Parry JV, Saunders NA, Nathan M, Soldan K, Szarewski A, Cuzick J, Beddows S (2011) Human papillomavirus genotype detection and viral load in paired genital and urine samples from both females and males. J Med Virol 83: 1744–1751.
Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V WHO International Agency for Research on Cancer Monograph Working Group (2009) A review of human carcinogens–Part B: biological agents. Lancet Oncol 10: 321–322.
Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, Kiviat N, Galloway DA (2000) Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis 181: 1911–1919.
Chin-Hong PV, Vittinghoff E, Cranston RD, Buchbinder S, Cohen D, Colfax G, Da Costa M, Darragh T, Hess E, Judson F, Koblin B, Madison M, Palefsky JM (2004) Age-specific prevalence of anal human papillomavirus infection in HIV-negative sexually active men who have sex with men: the EXPLORE study. J Infect Dis 190: 2070–2076.
Department of Health, United Kingdom Government (2014) HPV vaccination programme: change from 3 to 2 doses–Publications–GOV.UK. https://www.gov.uk/government/publications/schedule-change-from-3-to-2-doses-in-the-hpv-vaccination-programme . Accessed on: 11 November 2014.
Donà MG, Palamara G, Di Carlo A, Latini A, Vocaturo A, Benevolo M, Pimpinelli F, Giglio A, Moretto D, Impara G, Giuliani M (2012) Prevalence, genotype diversity and determinants of anal HPV infection in HIV-uninfected men having sex with men. J Clin Virol 54: 185–189.
Edelstein ZR, Carter JJ, Garg R, Winer RL, Feng Q, Galloway DA, Koutsky LA (2011) Serum antibody response following genital α9 human papillomavirus infection in young men. J Infect Dis 204: 209–216.
Frisch M, Smith E, Grulich A, Johansen C (2003) Cancer in a population-based cohort of men and women in registered homosexual partnerships. Am J Epidemiol 157: 966–972.
Giuliano A (2014) HPV seroconversion differs significantly by HPV type and anatomic site of infection among men: results from the HPV infection in men (HIM) study. PH.OA05.01. 29th International Papillomavirus Conference and Clinical & Public Health Workshops. Seattle. 216.
Giuliano AR, Lee J-H, Fulp W, Villa LL, Lazcano E, Papenfuss MR, Abrahamsen M, Salmeron J, Anic GM, Rollison DE, Smith D (2011a) Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet 377: 932–940.
Giuliano AR, Palefsky JM, Goldstone S, Moreira ED Jr, Penny ME, Aranda C, Vardas E, Moi H, Jessen H, Hillman R, Chang Y-H, Ferris D, Rouleau D, Bryan J, Marshall JB, Vuocolo S, Barr E, Radley D, Haupt RM, Guris D (2011b) Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 364: 401–411.
Health protection Agency (2014) Genitourinary Medicine Clinic Activity Dataset (GUMCADv2) http://www.hpa.org.uk/gumcad#Guidelines_and_specifications accessed on: 26 February 2014.
Hoots BE, Palefsky JM, Pimenta JM, Smith JS (2009) Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer 124: 2375–2383.
Jit M, Choi YH, Edmunds WJ (2008) Economic evaluation of human papillomavirus vaccination in the United Kingdom. BMJ 337: a769.
Joura E, Giuliano A, Iversen OE, Bautista O, Chen J, Moeller E, Ritter M, Luxembourg A (2014) Efficacy and immunogenicity of a novel 9-valent HPV L1 virus-like particle vaccine in 16-26 year old women [PH.OA02.01]. Programs and Abstracts of the 29th International Papillomavirus Conference and Clinical & Public Health Workshops, (Seattle).
Kim JJ (2010) Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis 10: 845–852.
Lacey HB, Wilson GE, Tilston P, Wilkins EG, Bailey AS, Corbitt G, Green PM (1999) A study of anal intraepithelial neoplasia in HIV positive homosexual men. Sex Transm Infect 75: 172–177.
Leval A, Herweijer E, Arnheim-Dahlström L, Walum H, Frans E, Sparén P, Simard JF (2012) Incidence of genital warts in Sweden before and after quadrivalent human papillomavirus vaccine availability. J Infect Dis 206: 860–866.
Machalek DA, Poynten M, Jin F, Fairley CK, Farnsworth A, Garland SM, Hillman RJ, Petoumenos K, Roberts J, Tabrizi SN, Templeton DJ, Grulich AE (2012) Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol 13: 487–500.
Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, PJF Snijders, Meijer CJLM International Agency for Research on Cancer Multicenter Cervical Cancer Study Group (2003) Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 348: 518–527.
Nyitray AG, Carvalho da Silva RJ, Baggio ML, Lu B, Smith D, Abrahamsen M, Papenfuss M, Villa LL, Lazcano-Ponce E, Giuliano AR (2011) Age-specific prevalence of and risk factors for anal human papillomavirus (HPV) among men who have sex with women and men who have sex with men: the HPV in men (HIM) study. J Infect Dis 203: 49–57.
Olsson S-E, van Damme P, Herrera T, Pitisutitthum P, Block S, Christiano S, Sun J, Luxembourg A (2014) Immunogenicity and safety of a novel 9-valent HPV L1 virus-like particle vaccine in boys and girls 9-15 years old, comparison to women 16-26 years old [abstract PH.PD04.03]. Program and Abstracts of the 29th International Papillomavirus Conference and Clinical & Public Health Workshops, (Seattle).
Palefsky JM, Giuliano AR, Goldstone S, Moreira ED Jr, Aranda C, Jessen H, Hillman R, Ferris D, Coutlee F, Stoler MH, Marshall JB, Radley D, Vuocolo S, Haupt RM, Guris D, Garner EIO (2011) HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 365: 1576–1585.
Parisi SG, Cruciani M, Scaggiante R, Boldrin C, Andreis S, Dal Bello F, Pagni S, Barelli A, Sattin A, Mengoli C, Palù G (2011) Anal and oral human papillomavirus (HPV) infection in HIV-infected subjects in northern Italy: a longitudinal cohort study among men who have sex with men. BMC Infect Dis 11: 150.
Pathak N, Dodds J, Zamora J, Khan K (2014) Accuracy of urinary human papillomavirus testing for presence of cervical HPV: systematic review and meta-analysis. BMJ 349: g5264–g5264.
Phanuphak N, Teeratakulpisarn N, Pankam T, Kerr SJ, Barisri J, Deesua A, Rodbamrung P, Hongchookiat P, Chomchey N, Phanuphak P, Sohn AH, Ananworanich J, Palefsky JM (2013) Anal human papillomavirus infection among Thai men who have sex with men with and without HIV infection: prevalence, incidence, and persistence. J Acquir Immune Defic Syndr 63: 472–479.
Quinn R, Salvatierra J, Solari V, Calderon M, Ton TGN, Zunt JR (2012) Human papillomavirus infection in men who have sex with men in Lima, Peru. AIDS Res Hum Retroviruses 28: 1734–1738.
De Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin H-R, Vallejos CS, de Ruiz PA, Lima MA, Guimera N, Clavero O, Alejo M, Llombart-Bosch A, Cheng-Yang C, Tatti SA, Kasamatsu E, Iljazovic E, Odida M, Prado R, Seoud M, Grce M, Usubutun A, Jain A, Suarez GAH, Lombardi LE, Banjo A, Menéndez C, Domingo EJ, Velasco J, Nessa A, Chichareon SCB, Qiao YL, Lerma E, Garland SM, Sasagawa T, Ferrera A, Hammouda D, Mariani L, Pelayo A, Steiner I, Oliva E, Meijer CJ, Al-Jassar WF, Cruz E, Wright TC, Puras A, Llave CL, Tzardi M, Agorastos T, Garcia-Barriola V, Clavel C, Ordi J, Andújar M, Castellsagué X, Sánchez GI, Nowakowski AM, Bornstein J, Muñoz N, Bosch FX (2010) Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 11: 1048–1056.
Sayers SJ, McMillan A, McGoogan E (1998) Anal cytological abnormalities in HIV-infected homosexual men. Int J STD AIDS 9: 37–40.
Schiller JT, Castellsagué X, Garland SM (2012) A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 30 (Suppl 5): F123–F138.
Toft L, Tolstrup M, Storgaard M, Østergaard L, Søgaard OS (2014) Vaccination against oncogenic human papillomavirus infection in HIV-infected populations: review of current status and future perspectives. Sex Health 11: 511–523.
Van der Snoek EM, Niesters HGM, van Doornum GJJ, Mulder PGH, ADME Osterhaus, van der Meijden WI (2005) Acquisition and clearance of perianal human papillomavirus infection in relation to HIV-positivity in men who have sex with men in the Netherlands. Acta Derm Venereol 85: 437–443.
Videla S, Darwich L, Cañadas M-P, Coll J, Piñol M, García-Cuyás F, Molina-Lopez RA, Cobarsi P, Clotet B, Sirera G HIV-HPV Study Group (2013) Natural history of human papillomavirus infections involving anal, penile, and oral sites among HIV-positive men. Sex Transm Dis 40: 3–10.
Sonnenberg P, Clifton S, Beddows S, Field N, Soldan K, Tanton C, Mercer CH, da Silva FC, Alexander S, Copas AJ, Phelps A, Erens B, Prah P, Macdowall W, Wellings K, Ison CA, Johnson AM (2013) Prevalence, risk factors, and uptake of interventions for sexually transmitted infections in Britain: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet 382: 1795–1806.
Swedish KA, Factor SH, Goldstone SE (2012) Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis 54: 891–898.
Swedish KA, Goldstone SE (2014) Prevention of anal condyloma with quadrivalent human papillomavirus vaccination of older men who have sex with men. PLoS One 9: e93393.
The FUTURE II Study Group (2007) Prophylactic efficacy of a quadrivalent human papillomavirus (HPV) vaccine in women with virological evidence of HPV infection. J Infect Dis 196: 1438–1446.
Wilkinson JR, Morris EJA, Downing A, Finan PJ, Aravani A, Thomas JD, Sebag-Montefiore D (2014) The rising incidence of anal cancer in England 1990–2010: a population-based study. Colorectal Dis 16: O234–O239.
Zou H, Tabrizi SN, Grulich AE, Garland SM, Hocking JS, Bradshaw CS, Morrow A, Prestage G, Cornall AM, Fairley CK, Chen MY (2014) Early acquisition of anogenital human papillomavirus among teenage men who have sex with men. J Infect Dis 209: 642–651.
Acknowledgements
We thank Natsal-3 for providing estimates for sexual behaviour in MSM, Cath Mercer for advice on questionnaire design, Andrew Copas for statistical advice, Graham Hart for advice during study planning, David Mesher for providing data from the Genitourinary Medicine Clinic Activity Dataset (GUMCAD) at Public Health England, which provided demographic estimates for MSM at STI clinics. WJE’s partner works for GSK. EMK was funded on a Medical Research Council (MRC) studentship. The study was supported in part by funds from the National Institute of Health Research (NIHR). There was no commercial funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Presented in part at the 29th Annual International Papillomavirus Conference, 21–25 August 2014, Seattle, Washington; Poster PH.PP02.41. Presented in part at the Public Health England Annual Conference 2014, 16–17 September 2014, Warwick University, UK; Poster 202.
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
King, E., Gilson, R., Beddows, S. et al. Human papillomavirus DNA in men who have sex with men: type-specific prevalence, risk factors and implications for vaccination strategies. Br J Cancer 112, 1585–1593 (2015). https://doi.org/10.1038/bjc.2015.90
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2015.90