Abstract
An earlier diagnosis is a key strategy for improving the outcomes of patients with cancer. However, achieving this goal can be challenging, particularly for the growing number of people with one or more chronic conditions (comorbidity/multimorbidity) at the time of diagnosis. Pre-existing chronic diseases might affect patient participation in cancer screening, help-seeking for new and/or changing symptoms and clinicians’ decision-making on the use of diagnostic investigations. Evidence suggests, for example, that pre-existing pulmonary, cardiovascular, neurological and psychiatric conditions are all associated with a more advanced stage of cancer at diagnosis. By contrast, hypertension and certain gastrointestinal and musculoskeletal conditions might be associated with a more timely diagnosis. In this Review, we propose a comprehensive framework that encompasses the effects of disease-specific, patient-related and health-care-related factors on the diagnosis of cancer in individuals with pre-existing chronic illnesses. Several previously postulated aetiological mechanisms (including alternative explanations, competing demands and surveillance effects) are integrated with newly identified mechanisms, such as false reassurances, or patient concerns about appearing to be a hypochondriac. By considering specific effects of chronic diseases on diagnostic processes and outcomes, tailored early diagnosis initiatives can be developed to improve the outcomes of the large proportion of patients with cancer who have pre-existing chronic conditions.
Key points
-
Many individuals with possible symptoms of cancer also have pre-existing chronic diseases, which can affect both diagnostic timeliness and cancer stage at diagnosis.
-
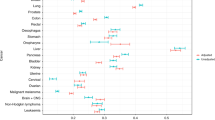
Evidence suggests that pulmonary, cardiovascular, neurological and psychiatric disorders are associated with longer intervals before cancer diagnosis and more advanced-stage disease at diagnosis.
-
Effects of specific chronic conditions seem to vary in terms of both direction and size according to pre-existing disease type and the nature of the presenting symptoms.
-
Targeted interventions designed to expedite cancer diagnosis and thus improve patient outcomes might be possible by considering the effects of chronic diseases on participation in cancer screening, patient help-seeking for cancer symptoms, and doctors’ decision-making about the use of investigations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hamilton, W., Walter, F. M., Rubin, G. & Neal, R. D. Improving early diagnosis of symptomatic cancer. Nat. Rev. Clin. Oncol. 13, 740–749 (2016).
Swann, R. et al. Diagnosing cancer in primary care: results from the National Cancer Diagnosis Audit. Br. J. Gen. Pract. 68, e63–e72 (2018).
Ritchie, C. S. et al. Association between patients’ perception of the comorbidity burden and symptoms in outpatients with common solid tumors. Cancer 123, 3835–3842 (2017).
Extermann, M. Interaction between comorbidity and cancer. Cancer Control 14, 13–22 (2007).
Sarfati, D., Koczwara, B. & Jackson, C. The impact of comorbidity on cancer and its treatment. CA Cancer J. Clin. 66, 337–350 (2016).
Klil-Drori, A. J., Azoulay, L. & Pollak, M. N. Cancer, obesity, diabetes, and antidiabetic drugs: is the fog clearing? Nat. Rev. Clin. Oncol. 14, 85–99 (2017).
Eibl, G. et al. Diabetes mellitus and obesity as risk factors for pancreatic cancer. J. Acad. Nutr. Diet. 118, 555–567 (2018).
Setiawan, V. W. et al. Pancreatic cancer following incident diabetes in African Americans and Latinos: the multiethnic cohort. J. Natl Cancer Inst. 111, 27–33 (2019).
Din, F. V. et al. Effect of aspirin and NSAIDs on risk and survival from colorectal cancer. Gut 59, 1670–1679 (2010).
Algra, A. M. & Rothwell, P. M. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 13, 518–527 (2012).
Onitilo, A. A. et al. Diabetes and cancer II: role of diabetes medications and influence of shared risk factors. Cancer Control 23, 991–1008 (2012).
Scott, S. E., Walter, F. M., Webster, A., Sutton, S. & Emery, J. The model of pathways to treatment: conceptualization and integration with existing theory. Br. J. Health Psychol. 18, 45–65 (2013).
Lyratzopoulos, G., Vedsted, P. & Singh, H. Understanding missed opportunities for more timely diagnosis of cancer in symptomatic patients after presentation. Br. J. Cancer 112 (Suppl. 1), S84–S91 (2015).
Walter, F., Webster, A., Scott, S. & Emery, J. The Andersen model of total patient delay: a systematic review of its application in cancer diagnosis. J. Health Serv. Res. Policy 17, 110–118 (2012).
Macdonald, S., Macleod, U., Campbell, N. C., Weller, D. & Mitchell, E. Systematic review of factors influencing patient and practitioner delay in diagnosis of upper gastrointestinal cancer. Br. J. Cancer 94, 1272–1280 (2006).
Mitchell, E., Macdonald, S., Campbell, N. C., Weller, D. & Macleod, U. Influences on pre-hospital delay in the diagnosis of colorectal cancer: a systematic review. Br. J. Cancer 98, 60–70 (2008).
Zhou, Y. et al. Diagnosis of cancer as an emergency: a critical review of current evidence. Nat. Rev. Clin. Oncol. 14, 45–56 (2017).
Corkum, M. et al. Impact of comorbidity and healthcare utilization on colorectal cancer stage at diagnosis: literature review. Cancer Control 23, 213–220 (2012).
The Academy of Medical Sciences. Multimorbidity: a priority for global health research. The Academy of Medical Sciences https://acmedsci.ac.uk/policy/policy-projects/multimorbidity (2019).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40, 373–383 (1987).
Geraci, J. M., Escalante, C. P., Freeman, J. L. & Goodwin, J. S. Comorbid disease and cancer: the need for more relevant conceptual models in health services research. J. Clin. Oncol. 23, 7399–7404 (2005).
Gurney, J., Sarfati, D. & Stanley, J. The impact of patient comorbidity on cancer stage at diagnosis. Br. J. Cancer 113, 1375–1380 (2015).
Gonzalez, E. C., Ferrante, J. M., Van Durme, D. J., Pal, N. & Roetzheim, R. G. Comorbid illness and the early detection of cancer. South. Med. J. 94, 913–920 (2001).
El-Charnoubi, W. A., Svendsen, J. B., Tange, U. B. & Kroman, N. Women with inoperable or locally advanced breast cancer — what characterizes them? A retrospective review of 157 cases. Acta Oncol. 51, 1081–1085 (2012).
Fleming, S. T., Pursley, H. G., Newman, B., Pavlov, D. & Chen, K. Comorbidity as a predictor of stage of illness for patients with breast cancer. Med. Care 43, 132–140 (2005).
O’Rourke, R. W. et al. Psychiatric illness delays diagnosis of esophageal cancer. Dis. Esophagus 21, 416–421 (2008).
Siddiqui, A. A. et al. Elevated HbA1c is an independent predictor of aggressive clinical behavior in patients with colorectal cancer: a case-control study. Dig. Dis. Sci. 53, 2486–2494 (2008).
Xiao, H. et al. Impact of comorbidities on prostate cancer stage at diagnosis in Florida. Am. J. Mens Health 10, 285–295 (2016).
Yasmeen, S. et al. Risk of advanced-stage breast cancer among older women with comorbidities. Cancer Epidemiol. Biomarkers Prev. 21, 1510–1519 (2012).
Desai, M. M., Bruce, M. L. & Kasl, S. V. The effects of major depression and phobia on stage at diagnosis of breast cancer. Int. J. Psychiatry Med. 29, 29–45 (1999).
Fleming, S. T., McDavid, K., Pearce, K. & Pavlov, D. Comorbidities and the risk of late-stage prostate cancer. ScientificWorldJournal 6, 2460–2470 (2006).
Gornick, M. E., Eggers, P. W. & Riley, G. F. Associations of race, education, and patterns of preventive service use with stage of cancer at time of diagnosis. Health Serv. Res. 39, 1403–1427 (2004).
Reid, B. C., Warren, J. L. & Rozier, G. Comorbidity and early diagnosis of head and neck cancer in a Medicare population. Am. J. Prev. Med. 27, 373–378 (2004).
Sikka, V. & Ornato, J. P. Cancer diagnosis and outcomes in Michigan EDs versus other settings. Am. J. Emerg. Med. 30, 283–292 (2012).
Taneja, S., Mandayam, S., Kayani, Z. Z., Kuo, Y. F. & Shahinian, V. B. Comparison of stage at diagnosis of cancer in patients who are on dialysis versus the general population. Clin. J. Am. Soc. Nephrol. 2, 1008–1013 (2007).
Baillargeon, J. et al. Effect of mental disorders on diagnosis, treatment, and survival of older adults with colon cancer. J. Am. Geriatr. Soc. 59, 1268–1273 (2011).
Ahn, D. H. et al. Influence of medical comorbidities on the presentation and outcomes of stage I–III non-small-cell lung cancer. Clin. Lung Cancer 14, 644–650 (2013).
Abgrall-Barbry, G. et al. Depressive mood and subsequent cancer diagnosis in patients undergoing a colonoscopy. Psychosomatics 53, 356–362 (2012).
Fazio, L., Cotterchio, M., Manno, M., McLaughlin, J. & Gallinger, S. Association between colonic screening, subject characteristics, and stage of colorectal cancer. Am. J. Gastroenterol. 100, 2531–2539 (2005).
Fisher, D. A. et al. Risk factors for advanced disease in colorectal cancer. Am. J. Gastroenterol. 99, 2019–2024 (2004).
Fisher, D. A. et al. Determinants of medical system delay in the diagnosis of colorectal cancer within the Veteran Affairs Health System. Dig. Dis. Sci. 55, 1434–1441 (2010).
Gupta, S. K. & Lamont, E. B. Patterns of presentation, diagnosis, and treatment in older patients with colon cancer and comorbid dementia. J. Am. Geriatr. Soc. 52, 1681–1687 (2004).
Iritani, S., Tohgi, M., Miyata, H. & Ohi, G. Impact of dementia on cancer discovery and pain. Psychogeriatrics 11, 6–13 (2011).
Henry, K. A., Sherman, R. & Roche, L. M. Colorectal cancer stage at diagnosis and area socioeconomic characteristics in New Jersey. Health Place 15, 505–513 (2009).
Raval, A. D., Madhavan, S., Mattes, M. D. & Sambamoorthi, U. Association between types of chronic conditions and cancer stage at diagnosis among elderly Medicare beneficiaries with prostate cancer. Popul. Health Manag. 19, 445–453 (2016).
Zafar, S. Y. et al. Comorbidity, age, race and stage at diagnosis in colorectal cancer: a retrospective, parallel analysis of two health systems. BMC Cancer 8, 345 (2008).
Elliss-Brookes, L. et al. Routes to diagnosis for cancer — determining the patient journey using multiple routine data sets. Br. J. Cancer 107, 1220–1226 (2012).
McPhail, S. et al. Emergency presentation of cancer and short-term mortality. Br. J. Cancer 109, 2027–2034 (2013).
Salika, T. et al. Associations between diagnostic pathways and care experience in colorectal cancer: evidence from patient-reported data. Frontline Gastroenterol. 9, 241–248 (2018).
Pruitt, S. L., Davidson, N. O., Gupta, S., Yan, Y. & Schootman, M. Missed opportunities: racial and neighborhood socioeconomic disparities in emergency colorectal cancer diagnosis and surgery. BMC Cancer 14, 927 (2014).
Sikka, V. Cancer diagnosis and outcomes in Michigan emergency departments versus other settings [abstract 278]. Ann. Emerg. Med. 56, S92 (2010).
Rabeneck, L., Paszat, L. F. & Li, C. Risk factors for obstruction, perforation, or emergency admission at presentation in patients with colorectal cancer: a population-based study. Am. J. Gastroenterol. 101, 1098–1103 (2006).
Askari, A. et al. Defining characteristics of patients with colorectal cancer requiring emergency surgery. Int. J. Colorectal Dis. 30, 1329–1336 (2015).
Wallace, D. et al. Identifying patients at risk of emergency admission for colorectal cancer. Br. J. Cancer 111, 577–580 (2014).
Beckett, P., Tata, L. & Hubbard, R. Risk factors and survival outcome for non-elective referral in non-small cell lung cancer patients — analysis based on the National Lung Cancer Audit. Lung Cancer 83, 396–400 (2014).
Markar, S. R. et al. Emergency presentation of esophagogastric cancer: predictors and long-term prognosis. Ann. Surg. 267, 711–715 (2018).
Mitchell, E., Rubin, G., Merriman, L. & Macleod, U. The role of primary care in cancer diagnosis via emergency presentation: qualitative synthesis of significant event reports. Br. J. Cancer 112 (Suppl. 1), S50–S56 (2015).
Shawihdi, M. et al. Variation in gastroscopy rate in English general practice and outcome for oesophagogastric cancer: retrospective analysis of Hospital Episode Statistics. Gut 63, 250–261 (2014).
Solsky, I. et al. Gastric cancer diagnosis after presentation to the ED: the independent association of presenting location and outcomes. Am. J. Surg. 216, 286–292 (2017).
Tsang, C., Bottle, A., Majeed, A. & Aylin, P. Cancer diagnosed by emergency admission in England: an observational study using the general practice research database. BMC Health Serv. Res. 13, 308 (2013).
Vajdic, C. M. et al. Health service utilisation and investigations before diagnosis of cancer of unknown primary (CUP): a population-based nested case-control study in Australian Government Department of Veterans’ Affairs clients. Cancer Epidemiol. 39, 585–592 (2015).
Renzi, C., Lyratzopoulos, G., Hamilton, W., Maringe, C. & Rachet, B. Contrasting effects of comorbidities on emergency colon cancer diagnosis: a longitudinal data-linkage study in England. BMC Health Serv. Res. 19, 311 (2019).
Black, G. et al. Patients’ experiences of cancer diagnosis as a result of an emergency presentation: a qualitative study. PLOS ONE 10, e0135027 (2015).
Gunnarsson, H., Holm, T., Ekholm, A. & Olsson, L. I. Emergency presentation of colon cancer is most frequent during summer. Colorectal Dis. 13, 663–668 (2011).
Mitchell, A. D., Inglis, K. M., Murdoch, J. M. & Porter, G. A. Emergency room presentation of colorectal cancer: a consecutive cohort study. Ann. Surg. Oncol. 14, 1099–1104 (2007).
Gunnarsson, H. et al. Heterogeneity of colon cancer patients reported as emergencies. World J. Surg. 38, 1819–1826 (2014).
Guilcher, S. J. et al. Level of disability, multi-morbidity and breast cancer screening: does severity matter? Prev. Med. 67, 193–198 (2014).
Lofters, A. et al. Screening for cervical cancer in women with disability and multimorbidity: a retrospective cohort study in Ontario, Canada. CMAJ Open 2, E240–E247 (2014).
Kiefe, C. I., Funkhouser, E., Fouad, M. N. & May, D. S. Chronic disease as a barrier to breast and cervical cancer screening. J. Gen. Intern. Med. 13, 357–365 (1998).
Klabunde, C. N. et al. Influence of age and comorbidity on colorectal cancer screening in the elderly. Am. J. Preventive Med. 51, e67–e75 (2016).
Zhao, G., Ford, E. S., Ahluwalia, I. B., Li, C. & Mokdad, A. H. Prevalence and trends of receipt of cancer screenings among US women with diagnosed diabetes. J. Gen. Intern. Med. 24, 270–275 (2009).
Bell, R. A., Shelton, B. J. & Paskett, E. D. Colorectal cancer screening in North Carolina: associations with diabetes mellitus and demographic and health characteristics. Prev. Med. 32, 163–167 (2001).
McBean, A. M. & Yu, X. The underuse of screening services among elderly women with diabetes. Diabetes Care 30, 1466–1472 (2007).
von Wagner, C. et al. Predictors of intention translation in bowel scope (flexible sigmoidoscopy) screening for colorectal cancer. Health Psychol. (in the press).
Chan, W. et al. Impact of socio-economic status on breast cancer screening in women with diabetes: a population-based study. Diabet. Med. 31, 806–812 (2014).
Lipscombe, L. L., Hux, J. E. & Booth, G. L. Reduced screening mammography among women with diabetes. Arch. Intern. Med. 165, 2090–2095 (2005).
Kendall, C. E. et al. A cross-sectional population-based study of breast cancer screening among women with HIV in Ontario, Canada. CMAJ Open 5, E673–E681 (2017).
Vigod, S. N., Kurdyak, P. A., Stewart, D. E., Gnam, W. H. & Goering, P. N. Depressive symptoms as a determinant of breast and cervical cancer screening in women: a population-based study in Ontario, Canada. Arch. Womens Mental Health 14, 159–168 (2011).
Martin-Lopez, R. et al. Inequalities in uptake of breast cancer screening in Spain: analysis of a cross-sectional national survey. Public Health 127, 822–827 (2013).
Wee, C. C., McCarthy, E. P., Davis, R. B. & Phillips, R. S. Obesity and breast cancer screening. J. Gen. Intern. Med. 19, 324–331 (2004).
Ferrante, J. M., Chen, P. H. & Jacobs, A. Breast and cervical cancer screening in obese minority women. J. Womens Health 15, 531–541 (2006).
Birt, L. et al. Responding to symptoms suggestive of lung cancer: a qualitative interview study. BMJ Open Respir. Res. 1, e000067 (2014).
Corner, J., Hopkinson, J. & Roffe, L. Experience of health changes and reasons for delay in seeking care: a UK study of the months prior to the diagnosis of lung cancer. Soc. Sci. Med. 62, 1381–1391 (2006).
Teppo, H. & Alho, O. P. Comorbidity and diagnostic delay in cancer of the larynx, tongue and pharynx. Oral Oncol. 45, 692–695 (2009).
Van Hout, A. M., de Wit, N. J., Rutten, F. H. & Peeters, P. H. Determinants of patient’s and doctor’s delay in diagnosis and treatment of colorectal cancer. Eur. J. Gastroenterol. Hepatol. 23, 1056–1063 (2011).
Brandenbarg, D. et al. Possible missed opportunities for diagnosing colorectal cancer in Dutch primary care: a multimethods approach. Br. J. Gen. Pract. 68, e54–e62 (2018).
Mor, V., Masterson-Allen, S., Goldberg, R., Guadagnoli, E. & Wool, M. S. Pre-diagnostic symptom recognition and help seeking among cancer patients. J. Commun. Health 15, 253–266 (1990).
McLachlan, S. et al. Symptom perceptions and help-seeking behaviour prior to lung and colorectal cancer diagnoses: a qualitative study. Fam. Pract. 32, 568–577 (2015).
Renzi, C., Whitaker, K. L., Winstanley, K., Cromme, S. & Wardle, J. Unintended consequences of an ‘all-clear’ diagnosis for potential cancer symptoms: a nested qualitative interview study with primary care patients. Br. J. Gen. Pract. 66, e158–e170 (2016).
Salika, T., Lyratzopoulos, G., Whitaker, K. L., Waller, J. & Renzi, C. Do comorbidities influence help-seeking for cancer alarm symptoms? A population-based survey in England. J. Public Health 40, 340–349 (2017).
Smith, S. M. et al. Factors contributing to the time taken to consult with symptoms of lung cancer: a cross-sectional study. Thorax 64, 523–531 (2009).
Cunningham, Y. et al. Symptom appraisal of potential lung cancer symptoms among people with chronic obstructive pulmonary disease — the challenge of multimorbidity. Psychooncology 28, 718–725 (2019).
Burgess, C. C., Ramirez, A. J., Smith, P. & Richards, M. A. Do adverse life events and mood disorders influence delayed presentation of breast cancer? J. Psychosom. Res. 48, 171–175 (2000).
Robinson, K., Christensen, K., Ottesen, B. & Krasnik, A. Socio-demographic factors, comorbidity and diagnostic delay among women diagnosed with cervical, endometrial or ovarian cancer. Eur. J. Cancer Care 20, 653–661 (2011).
Walter, F. M. et al. Symptoms and patient factors associated with longer time to diagnosis for colorectal cancer: results from a prospective cohort study. Br. J. Cancer 115, 533–541 (2016).
Cheung, D., Evison, F., Patel, P. & Trudgill, N. Factors associated with colorectal cancer occurrence after colonoscopy that did not diagnose colorectal cancer. Gastrointest. Endosc. 84, 287–295 (2016).
Nikonova, A., Guirguis, H. R., Buckstein, R. & Cheung, M. C. Predictors of delay in diagnosis and treatment in diffuse large B cell lymphoma and impact on survival. Br. J. Haematol. 168, 492–500 (2015).
Wagland, R. et al. Facilitating early diagnosis of lung cancer amongst primary care patients: the views of GPs. Eur. J. Cancer Care 26, e12704 (2017).
Mitchell, E. D., Rubin, G. & Macleod, U. Understanding diagnosis of lung cancer in primary care: qualitative synthesis of significant event audit reports. Br. J. Gen. Pract. 63, e37–e46 (2013).
Singh, H. et al. Missed opportunities to initiate endoscopic evaluation for colorectal cancer diagnosis. Am. J. Gastroenterol. 104, 2543–2554 (2009).
Bjerager, M., Palshof, T., Dahl, R., Vedsted, P. & Olesen, F. Delay in diagnosis of lung cancer in general practice. Br. J. Gen. Pract. 56, 863–868 (2006).
Mounce, L. T. A., Price, S., Valderas, J. M. & Hamilton, W. Comorbid conditions delay diagnosis of colorectal cancer: a cohort study using electronic primary care records. Br. J. Cancer 116, 1536–1543 (2017).
Walter, F. M. et al. Symptoms and other factors associated with time to diagnosis and stage of lung cancer: a prospective cohort study. Br. J. Cancer 112 (Suppl. 1), S6–S13 (2015).
Allison, P., Franco, E. & Feine, J. Predictors of professional diagnostic delays for upper aerodigestive tract carcinoma. Oral Oncol. 34, 127–132 (1998).
Friese, C. R. et al. Diagnostic delay and complications for older adults with multiple myeloma. Leuk. Lymphoma 50, 392–400 (2009).
Friese, C. R. et al. Timeliness and quality of diagnostic care for medicare recipients with chronic lymphocytic leukemia. Cancer 117, 1470–1477 (2011).
Huo, Q. et al. Delay in diagnosis and treatment of symptomatic breast cancer in China. Ann. Surg. Oncol. 22, 883–888 (2015).
Shah, H. A., Paszat, L. F., Saskin, R., Stukel, T. A. & Rabeneck, L. Factors associated with incomplete colonoscopy: a population-based study. Gastroenterology 132, 2297–2303 (2007).
Robertson, D. J. et al. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut 63, 949–956 (2014).
Chopra, D. & Hookey, L. C. Comorbid illness, bowel preparation, and logistical constraints are key reasons for outpatient colonoscopy nonattendance. Can. J. Gastroenterol. Hepatol. 2016, 2179354 (2016).
Koido, S. et al. Factors associated with incomplete colonoscopy at a Japanese academic hospital. World J. Gastroenterol. 20, 6961–6967 (2014).
Mills, K. et al. Understanding symptom appraisal and help-seeking in people with symptoms suggestive of pancreatic cancer: a qualitative study. BMJ Open 7, e015682 (2017).
Newschaffer, C. J. et al. Does comorbid disease interact with cancer? An epidemiologic analysis of mortality in a cohort of elderly breast cancer patients. J. Gerontol. A Biol. Sci. Med. Sci. 53, M372–M378 (1998).
Giovannucci, E. et al. Diabetes and cancer: a consensus report. Diabetes Care 33, 1674–1685 (2010).
Simon, T. G. et al. Diabetes, metabolic comorbidities, and risk of hepatocellular carcinoma: results from two prospective cohort studies. Hepatology 67, 1797–1806 (2018).
Hall, N. et al. Concerns, perceived need and competing priorities: a qualitative exploration of decision-making and non-participation in a population-based flexible sigmoidoscopy screening programme to prevent colorectal cancer. BMJ Open 6, e012304 (2016).
Whitaker, K. L., Macleod, U., Winstanley, K., Scott, S. E. & Wardle, J. Help seeking for cancer ‘alarm’ symptoms: a qualitative interview study of primary care patients in the UK. Br. J. Gen. Pract. 65, e96–e105 (2015).
Forbes, L. J. et al. Differences in cancer awareness and beliefs between Australia, Canada, Denmark, Norway, Sweden and the UK (the International Cancer Benchmarking Partnership): do they contribute to differences in cancer survival? Br. J. Cancer 108, 292–300 (2013).
Cassell, A. et al. The epidemiology of multimorbidity in primary care: a retrospective cohort study. Br. J. Gen. Pract. 68, e245–e251 (2018).
Driver, J. A. et al. Inverse association between cancer and Alzheimer’s disease: results from the Framingham Heart Study. BMJ 344, e1442 (2012).
NHS Digital. Adult psychiatric morbidity in England — 2007, results of a household survey. NHS https://digital.nhs.uk/data-and-information/publications/statistical/adult-psychiatric-morbidity-survey/adult-psychiatric-morbidity-in-england-2007-results-of-a-household-survey (2009).
World Health Organization. Data and resources. WHO www.euro.who.int/en/health-topics/noncommunicable-diseases/mental-health/data-and-resources (2018).
Solbjor, M., Skolbekken, J. A., Saetnan, A. R., Hagen, A. I. & Forsmo, S. Could screening participation bias symptom interpretation? An interview study on women’s interpretations of and responses to cancer symptoms between mammography screening rounds. BMJ Open 2, e001508 (2012).
Bandura, A. Health promotion from the perspective of social cognitive theory. Psychol. Health 13, 623–649 (1998).
Singh, H. & Graber, M. L. Improving diagnosis in health care — the next imperative for patient safety. N. Engl. J. Med. 373, 2493–2495 (2015).
Singh, H. & Sittig, D. F. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual. Saf. 24, 103–110 (2015).
Murphy, D. R. et al. Electronic health record-based triggers to detect potential delays in cancer diagnosis. BMJ Qual. Saf. 23, 8–16 (2014).
Salisbury, C. et al. Management of multimorbidity using a patient-centred care model: a pragmatic cluster-randomised trial of the 3D approach. Lancet 392, 41–50 (2018).
Fuller, E., Fitzgerald, K. & Hiom, S. Accelerate, Coordinate, Evaluate Programme: a new approach to cancer diagnosis. Br. J. Gen. Pract. 66, 176–177 (2016).
Moseholm, E. & Lindhardt, B. O. Patient characteristics and cancer prevalence in the Danish cancer patient pathway for patients with serious non-specific symptoms and signs of cancer — a nationwide, population-based cohort study. Cancer Epidemiol. 50, 166–172 (2017).
Naeser, E., Fredberg, U., Moller, H. & Vedsted, P. Clinical characteristics and risk of serious disease in patients referred to a diagnostic centre: a cohort study. Cancer Epidemiol. 50, 158–165 (2017).
Foster, A., Renzi, C. & Lyratzopoulos, G. Diagnosing cancer in patients with ‘non-alarm’ symptoms: learning from diagnostic care innovations in Denmark. Cancer Epidemiol. 54, 101–103 (2018).
Nicholson, B. D. et al. The Suspected CANcer (SCAN) pathway: protocol for evaluating a new standard of care for patients with non-specific symptoms of cancer. BMJ Open 8, e018168 (2018).
Fisher, D. A., Judd, L. & Sanford, N. S. Inappropriate colorectal cancer screening: findings and implications. Am. J. Gastroenterol. 100, 2526–2530 (2005).
Grunfeld, E. et al. Improving chronic disease prevention and screening in primary care: results of the BETTER pragmatic cluster randomized controlled trial. BMC Fam. Pract. 14, 175 (2013).
Mazza, D. & Mitchell, G. Cancer, ageing, multimorbidity and primary care. Eur. J. Cancer Care 26, e12717 (2017).
Almond, S., Mant, D. & Thompson, M. Diagnostic safety-netting. Br. J. Gen. Pract. 59, 872–874 (2009).
Sheringham, J. et al. Variations in GPs’ decisions to investigate suspected lung cancer: a factorial experiment using multimedia vignettes. BMJ Qual. Saf. 26, 449–459 (2017).
Meyer, A. D., Payne, V. L., Meeks, D. W., Rao, R. & Singh, H. Physicians’ diagnostic accuracy, confidence, and resource requests: a vignette study. JAMA Intern. Med. 173, 1952–1958 (2013).
Bhise, V. et al. Patient perspectives on how physicians communicate diagnostic uncertainty: an experimental vignette study. Int. J. Qual. Health Care 30, 2–8 (2018).
Bhise, V. et al. Defining and measuring diagnostic uncertainty in medicine: a systematic review. J. Gen. Intern. Med. 33, 103–115 (2018).
Erichsen, R., Horvath-Puho, E., Iversen, L. H., Lash, T. L. & Sorensen, H. T. Does comorbidity interact with colorectal cancer to increase mortality? A nationwide population-based cohort study. Br. J. Cancer 109, 2005–2013 (2013).
Iversen, L. H., Norgaard, M., Jacobsen, J., Laurberg, S. & Sorensen, H. T. The impact of comorbidity on survival of Danish colorectal cancer patients from 1995 to 2006 — a population-based cohort study. Dis. Colon Rectum. 52, 71–78 (2009).
Berger, Z. D. et al. Patient centred diagnosis: sharing diagnostic decisions with patients in clinical practice. BMJ 359, j4218 (2017).
Lawal, A. K. et al. What is a clinical pathway? Refinement of an operational definition to identify clinical pathway studies for a Cochrane systematic review. BMC Med. 14, 35 (2016).
The National Institute for Health and Care Excellence. NICE pathways: what are NICE pathways? NICE www.nice.org.uk/about/what-we-do/our-programmes/about-nice-pathways (2019).
Weller, D. et al. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br. J. Cancer 106, 1262–1267 (2012).
Jaen, C. R., Stange, K. C. & Nutting, P. A. Competing demands of primary care: a model for the delivery of clinical preventive services. J. Fam. Pract. 38, 166–171 (1994).
Feinstein, A. R. The pre-therapeutic classification of co-morbidity in chronic disease. J. Chronic Dis. 23, 455–468 (1970).
Pace, R. et al. Testing the reliability and efficiency of the pilot Mixed Methods Appraisal Tool (MMAT) for systematic mixed studies review. Int. J. Nurs. Stud. 49, 47–53 (2012).
Braithwaite, D. et al. Screening outcomes in older US women undergoing multiple mammograms in community practice: does interval, age, or comorbidity score affect tumor characteristics or false positive rates? J. Natl Cancer Inst. 105, 334–341 (2013).
Walter, L. C. et al. Impact of age and comorbidity on colorectal cancer screening among older veterans. Ann. Intern. Med. 150, 465–473 (2009).
Jiménez-Garcia, R., Hernandez-Barrera, V., Carrasco-Garrido, P. & Gil, A. Prevalence and predictors of breast and cervical cancer screening among Spanish women with diabetes. Diabetes Care 32, 1470–1472 (2009).
Cunningham, R., Sarfati, D., Stanley, J., Peterson, D. & Collings, S. Cancer survival in the context of mental illness: a national cohort study. Gen. Hosp. Psychiatry 37, 501–506 (2015).
Acknowledgements
This research arises from the CanTest Collaborative, which is funded by Cancer Research UK (C8640/A23385). C.R. acknowledges funding from a BMA TP Gunton research grant. H.S. is partly supported by the VA Health Services Research and Development Service Center for Innovations in Quality, Effectiveness and Safety (CIN13-413). G.L. acknowledges funding from Cancer Research UK (Advanced Clinician Scientist Fellowship Award, grant number C18081/A18180). J.E. acknowledges funding from an NHMRC Practitioner Fellowship.
Review criteria
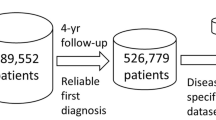
This Review includes original research involving quantitative, qualitative and mixed methods. Qualitative studies are included in order to provide insights into the complex effects of chronic conditions and their underlying mechanisms. The available evidence refers to cohort (n = 31), cross-sectional (n = 25) and case-control (n = 6) studies, as well as case-series (n = 13) and qualitative studies (n = 11). A quality score was assigned to each reference according to the Mixed Methods Appraisal Tool (MMAT) (further details on the review methods are provided in the Supplementary Box and Figure). The MMAT is a validated quality assessment tool, enabling the merits of each study to be evaluated based on various criteria specific for the different study designs (with a highest possible score of 100, if all criteria are met). Most studies received a MMAT score of 75 or 50 (35 and 31 studies, respectively); a score of 100 was assigned to 11 studies; and only 1 study received a score of 25 (Supplementary Table 1).
Author information
Authors and Affiliations
Contributions
C.R. and A.K researched data for the manuscript. All authors made a substantial contribution to discussions of content. C.R. and G.L. wrote the manuscript. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Renzi, C., Kaushal, A., Emery, J. et al. Comorbid chronic diseases and cancer diagnosis: disease-specific effects and underlying mechanisms. Nat Rev Clin Oncol 16, 746–761 (2019). https://doi.org/10.1038/s41571-019-0249-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-019-0249-6
This article is cited by
-
Implementation and external validation of the Cambridge Multimorbidity Score in the UK Biobank cohort
BMC Medical Research Methodology (2024)
-
Factors associated with the melanoma diagnostic interval in Ontario, Canada: a population-based study
British Journal of Cancer (2024)
-
Team complexity and care coordination for cancer survivors with multiple chronic conditions: a mixed methods study
Journal of Cancer Survivorship (2024)
-
Body mass index and cervical cancer screening among women aged 15–69 years in Eswatini: evidence from a population-based survey
BMC Public Health (2023)
-
Comparing variants related to chronic diseases from genome-wide association study (GWAS) and the cancer genome atlas (TCGA)
BMC Medical Genomics (2023)