-
PDF
- Split View
-
Views
-
Cite
Cite
L. M. A. J. Muller, K. J. Gorter, E. Hak, W. L. Goudzwaard, F. G. Schellevis, A. I. M. Hoepelman, G. E. H. M. Rutten, Increased Risk of Common Infections in Patients with Type 1 and Type 2 Diabetes Mellitus, Clinical Infectious Diseases, Volume 41, Issue 3, 1 August 2005, Pages 281–288, https://doi.org/10.1086/431587
Close - Share Icon Share
Abstract
Background. Clinical data on the association of diabetes mellitus with common infections are virtually lacking, not conclusive, and often biased. We intended to determine the relative risks of common infections in patients with type 1 and type 2 diabetes mellitus (DM1 and DM2, respectively).
Methods. In a 12-month prospective cohort study conducted as part of the Second Dutch National Survey of General Practice, we compared 705 adult patients who had DM1 and 6712 adult patients who had DM2 with 18,911 control patients who had hypertension without diabetes. Outcome measures were medically attended episodes of infection of the respiratory tract, urinary tract, and skin and mucous membranes. We applied multivariable and polytomous logistic regression analysis to determine independent risks of infections and their recurrences in patients with diabetes, compared with control patients.
Results. Upper respiratory infections were equally common among patients with diabetes and control patients. Patients with diabetes had a greater risk of lower respiratory tract infection (for patients with DM1: adjusted odds ratio [AOR], 1.42 [95% confidence interval {CI}, 0.96–2.08]; for patients with DM2: AOR, 1.32 [95% CI, 1.13–1.53]), urinary tract infection (for patients with DM1: AOR, 1.96 [95% CI, 1.49–2.58]; for patients with DM2: AOR, 1.24 [95% CI, 1.10–1.39]), bacterial skin and mucous membrane infection (for patients with DM1: AOR, 1.59 [95% CI, 1.12–2.24]; for patients with DM2: AOR, 1.33 [95% CI, 1.15–1.54]), and mycotic skin and mucous membrane infection (for patients with DM1: AOR, 1.34 [95% CI, 0.97–1.84]; for patients with DM2: AOR, 1.44 [95% CI, 1.27–1.63]). Risks increased with recurrences of common infections.
Conclusions. Patients with DM1 and DM2 are at increased risk for lower respiratory tract infection, urinary tract infection, and skin and mucous membrane infection. Studies are warranted into management of such infections in patients with diabetes.
Diabetes mellitus (DM) has been associated with increased rates of infections [1–3], which may be partially explained by a decreased T cell–mediated immune response [2, 3]. Impaired neutrophil function associated with diabetes has also been documented [4], although this is currently being debated [5]. Evidence from clinical studies for a causal relation between diabetes and common infections is, however, limited and not consistent [1, 6]. Some studies have shown that both common and rare infections are more prevalent among patients with diabetes than among the general population, whereas other studies have not observed such an association [2, 3, 7]. Patients with diabetes appeared to have an increased risk of asymptomatic bacteriuria and urinary tract infection [6–9] and of skin and mucous membrane infection, including Candida infections and infections of the foot [2, 3, 10]. To date, the relationship between respiratory tract infection and diabetes remains unclear [2, 10–12].
One of the explanations for the controversial data on diabetes and common infections might be that both selection and confounding bias may have distorted the observed associations [1]. For example, in a recent, large, controlled cohort study by Shah et al. [1], the observed differences may have been caused by differences in care-seeking behavior, and comorbidity was not taken into account. Some other studies were designed as a case-control studies, which typically risk a severe bias caused by selection of control subjects [6, 7]. In addition, most studies were hospital-based and did not account for differences in type of diabetes mellitus. The American Diabetes Association recently issued guidelines on influenza and pneumococcal immunization in diabetes [13, 14], but more-specific information on treatment of (common) infections is lacking. For example, guidelines of the American College of Family Physicians on respiratory tract infection do not mention a specific approach for patients with diabetes [15–18]. In The Netherlands, most patients with diabetes are treated in primary care, and infections are mainly managed by general practitioners. Therefore, the Second Dutch National Survey of General Practice provided an excellent opportunity to assess the independent risk of respiratory tract infection, urinary tract infection, and skin and mucous membrane infections in patients with type 1 and type 2 diabetes mellitus (DM1 and DM2, respectively).
Patients and Methods
A prospective cohort study was conducted using data from the Second Dutch National Survey of General Practice. In the survey, 195 general practitioners were trained to register all patients with whom they had contact during a 12-month period within the study period (from May 2000 through April 2002) (a total of 390,000 patients) [19]. Patient contacts were registered in computerized medical records with use of the International Classification of Primary Care (ICPC) codes [20] for diagnoses. Also, services administered by the general practitioner after the presented morbidity were recorded using codes of the Anatomical Therapeutical Chemical (ATC) classification index for drugs, and referrals were recorded, including type of care [21]. Detailed data from secondary care facilities were not recorded in a standardized way [19]. General practitioners received feedback about the quality of their registration (i.e., the number of contacts with an ICPC code). Ultimately, 93% of all patient contacts received an ICPC code (F. G. Schellevis, personal communication). Active medical comorbid conditions were considered to be present if a contact for such a disease was made in the year of registration. Patients aged ⩾18 years with DM1 or DM2 and control patients with hypertension but without diabetes were eligible for inclusion if they met the inclusion and exclusion criteria below.
Inclusion Criteria
Patients with DM1 and DM2. Patients with at least 1 patient contact with an ICPC code indicating DM1 (T90.1) or DM2 (T90.2) were selected. Because the majority of patients with DM1 are medically treated in both primary and secondary care facilities, for pragmatic reasons, we chose to further classify patients with a contact registered with ICPC codes T90.0 (diabetes not specified) or T90.3 (other diabetes) according to their age and medical management, as follows: patients <35 years of age were classified as having DM1. Patients aged ⩾35 years who used oral antidiabetic medication (ATC code A10B), with or without insulin (ATC code A10A), or who where referred to a dietician without any medication were classified as having DM2.
Control patients with hypertension. Patients with at least 1 contact for hypertension with (ICPC code K87) or without cardiovascular complications (K86) were selected for the control group. Therefore, no selection was made with regard to the presence or absence of active or inactive cardiovascular comorbidity as recorded in the medical records of the general practitioner. Guidelines of family physicians on the treatment of diabetes and hypertension recommend a 3-month follow-up period and control of both diseases [22–25]. Moreover, it is well known that hypertension does not carry a risk for common infections.
Exclusion Criteria
We excluded the following eligible patients who required a specific management of infections from the cohort: patients with other traumatic injuries of the respiratory tract (ICPC code R88), traumatic injuries of the kidney and urinary tract (U80), congenital abnormalities of the respiratory and urinary tracts (R89 and U85), and other rare diseases of the respiratory and urinary tracts (R99 and U99); patients with malignancies of the respiratory (ICPC codes R84 and R85) and urinary tracts (U75 and U76); patients with Hodgkin disease (ICPC code B72), leukemia (B73), malignant neoplasm of blood and lymph system (B74), and HIV infection (B90); patients using immunosuppressive medication (ATC codes L01, L02, and L04); and patients using ⩾10 prescriptions of corticosteroids (ATC code R03) or antibiotics (ATC code J01) during the 12-month period.
Measurements
Baseline risk factors. After an extensive literature search, we obtained information for each cohort member on possible risk factors that may confound the association between diabetes and infections. We took age and sex into account. In The Netherlands, all persons below a certain income level (€30,000 gross income per year in 2001) are automatically insured through the Sick Fund (∼60% of the Dutch population), and type of health insurance, therefore, is an accepted proxy for socioeconomic status. The presence of risk-elevating medical conditions was recorded by any contact for the following conditions and was assembled into large main categories for statistical reasons, as follows: pulmonary disease, including tuberculosis (ICPC code R70), acute bronchitis (R78), benign neoplasm (R86), chronic bronchitis (R91), emphysema/chronic obstructive pulmonary disease (R95), and asthma (R96); renal disease, including glomerular nephritis or nephrosis (U88) and urolithiasis (U95); incontinence of urine (U04); cardiovascular disease, including angina pectoris (K74), acute myocardial infarction (K75), ischemic heart disease (K76), cardiac decompensation (K77), atrial fibrillation (K78), paroxysmal tachycardia (K79), ectopic beats or extrasystole (K80), cor pulmonale (K82), nonrheumatic valve disease (K83), heart disease not specified (K84), cerebro vascular disease (K89 and K90), and peripheral artery disease (K92); peripheral neuropathy, including tingle of fingers, feet, or toes (N05) and peripheral neuritis (N94); vaginal disease, including vaginitis or vulvitis (X84) and prolapse of the uterus or vagina (X87); psychiatric disease, including organic psychosis (P71), schizophrenia (P72), affective psychosis (P73), and depression (P76); neurologic disease, including multiple sclerosis (N86), Parkinson disease (N87), and dementia (P70); and thyroid disease, including hyper- (T85) and hypothyreoidia (T86).
Outcome measures. Medically attended episodes of respiratory tract infection, urinary tract infection, and skin and mucous membrane infections were diagnosed and classified by the general practitioner. An episode could include 1 or more contacts with the general practitioner. A new episode was defined if a patient was free of signs or symptoms for a 30-day period. A second episode occurring >30 days after the initial episode was considered to be a recurrence. Respiratory tract infections were subdivided into upper and lower respiratory tract infections. Upper respiratory tract infection was defined as the occurrence of acute otitis media (ICPC code H71), streptococcal throat infection (R72), acute rhinolaryngitis (R74 and R77), sinusitis (R75), or acute tonsillitis or peritonsillar abscess (R76) according to a previous study performed by our group [26]. Lower respiratory tract infections included acute bronchitis (R78), influenza (R80), pneumonia (R81), pleuritis (R82), emphysema or chronic obstructive pulmonary disease (R95), and an exacerbation of asthma (R96). Urinary tract infections included acute pyelonephritis (U70), cystitis (U71), nonspecific urethritis (U72), and prostatitis (Y73). Skin and mucous membrane infections were divided into bacterial and mycotic infections. Bacterial skin and mucous membrane infections included otitis externa (H70), local infection not otherwise specified (S09), furuncle/abscess nose (R73), cellulitis (S10), nondefined infection of the skin (S76), and impetigo (S84). Mycotic skin and mucous membrane infections included dermatomycosis (S74), skin candida infections (S75), and urogenital candida infections (X72).
Statistical Analysis
Data were analyzed using SPSS software for Windows, version 11.0 (SPSS). The different outcomes were assembled into categories corresponding with an organ system in upper or lower respiratory tract infections, urinary tract infections, and bacterial and mycotic skin and mucous membrane infections and were analyzed as a dichotomous outcome (absence versus presence), as well as a class outcome (0, 1, and ⩾2 episodes). We applied multivariable logistic regression analysis to assess the independent risk of the separate infection categories adjusted for potential confounding variables. The following potential confounders were added to the regression equation for all infection categories: age, sex, health insurance type, and presence of high risk comorbid conditions (including pulmonary disease, cardiovascular disease, peripheral neuropathy, and neurologic disease). Urinary incontinence was added as a potential confounder in the multivariable analysis, with urinary tract infection as outcome variable. The multivariable analyses were conducted separately for patients with DM1 and DM2. Polytomous logistic regression analysis was used to determine independent risks of diabetes for the occurrence of 1 or ⩾2 episodes (recurrence). Such models use information contained in differences within categories, in differences between nonreference categories, and in ordering among categories. We finally conducted subgroup analyses for sex (male versus female) for the outcome urinary tract infection, the presence of cardiovascular disease (absent versus present), and use of insulin (yes or no). Adjusted odds ratios (AORs) and 95% CIs were given as an approximation of the relative risk. A P value <.05 was considered to be statistically significant.
Results
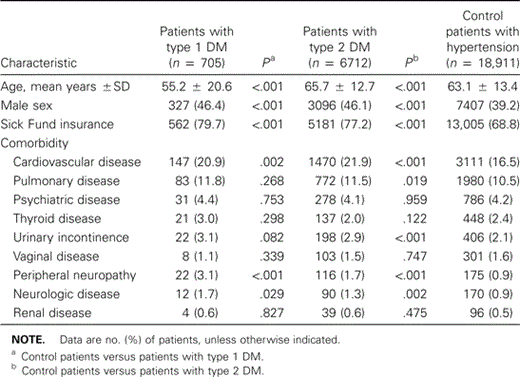
A total of 705 patients with DM1, 6712 patients with DM2, and 18,911 patients with hypertension were included in the analysis. Patients with DM1 were younger, and both patients with DM1 and those with DM2 were more often male and were more often insured through the Sick Fund, compared with control patients (table 1). In addition, all patients with diabetes had a higher prevalence of cardiovascular disease, peripheral neuropathy, and neurologic disease, and patients with DM2 had more instances of urinary incontinence and pulmonary disease, compared with control patients. Patients with DM2 were treated as follows: 110 (2%) of patients used insulin, 576 (9%) used both insulin and oral antidiabetic medication, 5139 (76%) used oral medication, and 13% did not use any glucose lowering medication (data not shown). The annual median consultation rate was comparable for patients with diabetes and control patients (patients with DM1, 7 consultations; patients with DM2, 9 consultations; control patients, 7 consultations).
Baseline characteristics of patients with type 1 and type 2 diabetes mellitus (DM) and control patients with hypertension.
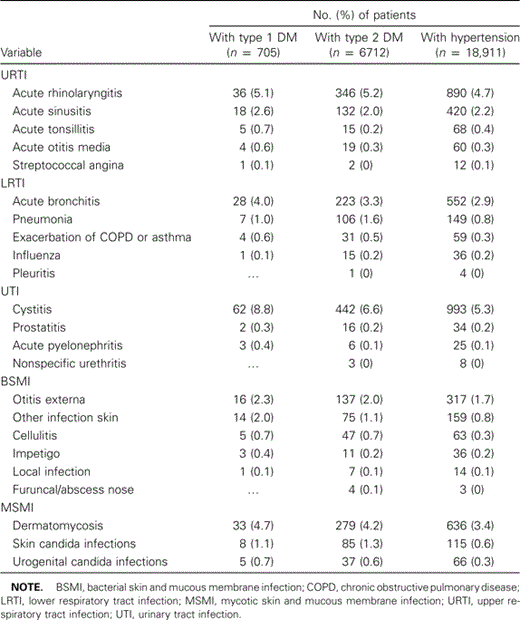
Cystitis, acute rhinolaryngitis, dermatomycoses, and acute bronchitis were the most common infections for which people attended their general practitioner, whereas pleuritis, abscess of the nose, and nonspecific urethritis were rare diseases (table 2). In all, the incidence of infections in the diabetes populations was equal to or greater than that in the control population.
Incidence of new episodes of infection in patients with type 1 and type 2 diabetes mellitus (DM) and control patients with hypertension.
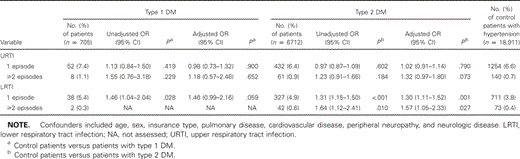
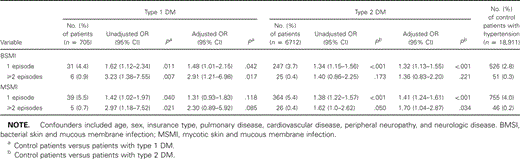
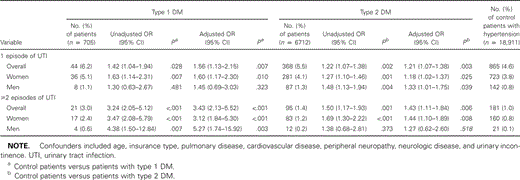
With use of multivariable logistic regression analysis, we found that patients with DM1 and DM2 had an increased relative risk for 1 or more episodes of lower respiratory tract infection (patients with DM1: AOR, 1.42 [95% CI, 0.96–2.08]; patients with DM2: AOR, 1.32 [95% CI, 1.13–1.53] and for urinary tract infection (patients with DM1: AOR, 1.96 [95% CI, 1.49–2.58]; patients with DM2: AOR, 1.24 [95% CI, 1.10–1.39]). Also, patients with DM1 and DM2 had an increased relative risk for bacterial skin and mucous membrane infections (patients with DM1: AOR, 1.59 [95% CI, 1.12–2.24]; patients with DM2: AOR, 1.33 [95% CI, 1.15–1.54]) and mycotic skin and mucous membrane infections (patients with DM1: AOR, 1.34 [95% CI, 0.97–1.84]; patients with DM2, AOR 1.44 [95% CI, 1.27–1.63]). We did not observe an increased risk for upper respiratory tract infection in patients with DM1 and DM2 (patients with DM1: AOR, 0.95 [95% CI, 0.72–1.26]; patients with DM2: AOR, 1.05 [95% CI, 0.95–1.18]) (data not shown).
Polytomous logistic regression analysis showed that the relative risk for recurrences of most common infections among patients with diabetes was higher than the relative risk for a single disease episode (tables 3–5). Multivariable analyses done separately for patients with and without cardiovascular disease, as well as for patients with and without use of insulin, showed no substantial differences in overall results for associations between diabetes and infections (data not shown).
Unadjusted and adjusted associations between diabetes mellitus (DM) and respiratory tract infections, determined by polytomous logistic regression analysis.
Unadjusted and adjusted associations between diabetes mellitus (DM) and skin and mucous membranes infections, determined by polytomous logistic regression analysis.
Discussion
Both patients with DM1 and those with DM2 are at increased risk for common infections (notably for recurrences). Our study has distinctive strengths. First, we studied a unique cohort of unselected populations with diabetes and with and without insulin use in primary care. Second, we were able to study associations of diabetes with common infections involving different organ systems. Third, the study had sufficient power to detect statistically significant associations. Finally, data were obtained from a large, nationally representative cohort study involving Dutch primary care facilities, and training of general practitioners and financial arrangements guaranteed a high-quality morbidity registration. Moreover, the diagnosed comorbidity is well registered due to the fact that, in The Netherlands, >90% of the patients have their own general practitioner who is the porte d'entrée for specialist care. Detailed recording of the ongoing medical history and patient characteristics of each patient were already available.
So far, the evidence for absence of risk-elevation of upper respiratory tract infection and increased risk for lower respiratory tract infection, as observed in our study, has been conflicting. A former study reported a higher risk of unspecified upper respiratory tract infection in patients with diabetes [1]. In other case-control and cohort studies, diabetes was not found to be associated with pneumonia [27–29]. These studies may be criticized, because elderly or chronically ill patients were included, and results may, therefore, not be applicable to the diabetes population as a whole.
For all women with diabetes, the risk of urinary tract infection was greater than in control patients, which is in accordance with the findings of most of the existing studies [6, 7, 30, 31]. However, former studies mainly included female patients with diabetes, and no associations were reported for men. We also found men at increased risk for urinary tract infection, especially among patients with DM1 (OR, 1.96). The higher incidence in men could mainly be explained by prostatitis. This study revealed that the relative risk for recurrences of urinary tract infection was especially high in women with type 1 DM (OR, 3.47). A higher level of recurrence of urinary tract infection has already been found, as described in a review by Patterson et al. [8], but studies were selected by including women with asymptomatic bacteriuria only.
There are several controlled studies documenting an increased risk of skin and mucous membrane infections in patients with diabetes [1, 32, 33]. Despite the fact that these studies could be criticized because of potentially uncontrolled selection and confounding bias, our study also showed that both patients with DM1 and those with DM2 are at increased risk for bacterial skin and mucous membrane infections and mycotic skin and mucous membrane infections. In particular, patients with DM1 had an increased risk for recurrences. This increased risk could be explained by a more-frequent carriage of Staphylococcus aureus found in insulin-dependent diabetic patients [34]. Otitis externa was one of the main infections in this group, and Shah et al. [1] also reported an increased risk of this infection in patients with DM. Mycotic skin and mucous membrane infection was predominantly determined by dermatomycosis. It has been shown that patients with diabetes are at increased risk of onychomycosis [35].
Current evidence of the association between diabetes and infections is derived from uncontrolled studies or studies in which control subjects were sampled from the general population. Both types of study designs may risk a severe selection bias, because patients with diabetes visit the physician regularly for control of their diabetes. It has, for example, been shown that the number of prior visits to a primary care physician increases the risk of serious respiratory tract infections during an influenza epidemic [14]. In contrast to studies performed thus far, we have selected patients with hypertension as the reference group, because they need a regular check-up, and there is no known elevated risk for common infections in this group. We observed similar consultation rates in control patients and patients with diabetes; therefore, selection bias in our study is unlikely. Moreover, data on the total population of the Second Dutch National Survey of General Practice showed that the incidences of common infections cystisis, dermatomycosis and acute bronchitis are 3.3%, 3.1%, and 2.2%, respectively [36]. Patients with hypertension in our study had more infections (5.3%, 3.4%, and 2.9%, respectively), which supports our assumptions. Some of the control patients may have had undiagnosed diabetes, which would have led to an underestimation of the true associations. However, the estimated prevalence of undiagnosed DM2 in patients with hypertension is ∼5% [37].
It might be argued that, for some study subjects, there may be possible misclassification of type of diabetes as a result of using an age cut off point of 35 years, notably for patients with maturity-onset diabetes of the young. According to Owen et al. [38], this condition affects only 1%–2% of patients with DM2. Only ∼20% of the subjects were possibly misclassified as having DM1 instead of DM2. As expected, subgroup analysis on the basis of insulin use did not, therefore, materially change the results. Also, some might argue that patients with diabetes and control patients were completely different with regard to other risk factors that may have confounded the observed association between diabetes and infections. However, we tried to minimize this type of bias by collecting information for each study subject on possible confounders, and we adjusted for known differences between the comparison groups using multivariable analysis. As shown in table 3–5, the small differences between the unadjusted ORs and AORs indicate that the confounding effect must have been small. Also, residual confounding resulting from differences in potentially unmeasured confounders is very unlikely. Smoking could, for example, have influenced the relation between diabetes and respiratory tract infections, but the smoking habits of the patients were unknown. However, data collected for September 2003 through August 2004 from the general practice research network of the Academic Medical Centre St. Radboud (Nijmegen, The Netherlands) [39] showed that there is no difference in smoking habits between patients with DM and those with hypertension (W. H. E. M. van Gerwen, personal communication). Because the incidence of new patients with DM is ∼10 times lower than the prevalence (26.3 cases per 1000 patients), it is very unlikely that preceding infections might have induced a diagnostic bias. Finally, it might be argued that we should have chosen more-narrow categories and subgroups of patients. Our main aim was to assess the independent risk of common infections in patients with diabetes. By design, we therefore had inadequate statistical power to analyze a large number of subgroups.
In conclusion, our study very clearly showed that both patients with DM1 and those with DM2 are at increased risk for common infections. This may affect prevention and treatment of infections in patients with DM. Therefore, studies of the management of infections in patients with DM are warranted.
Acknowledgments
We thank Mrs. Nicole Boekema for supporting in data management.
Financial support. Public Health Fund, The Hague, The Netherlands (grant U03/175-P230).
Potential conflicts of interest. All authors: no conflicts.
References
Figures and Tables
Unadjusted and adjusted associations between diabetes mellitus (DM) and urinary tract infections, determined by polytomous logistic regression analysis.





Comments