-
PDF
- Split View
-
Views
-
Cite
Cite
Verena Schneider, Linda E. Lévesque, Bin Zhang, Thomas Hutchinson, James M. Brophy, Association of Selective and Conventional Nonsteroidal Antiinflammatory Drugs with Acute Renal Failure: A Population-based, Nested Case-Control Analysis, American Journal of Epidemiology, Volume 164, Issue 9, 1 November 2006, Pages 881–889, https://doi.org/10.1093/aje/kwj331
Close - Share Icon Share
Abstract
Conventional nonsteroidal antiinflammatory drugs (NSAIDs) are associated with acute renal failure, but cyclooxygenase-2 inhibitors have not been comparatively evaluated. The authors conducted a nested case-control study to assess the association between exposure to NSAIDs, including cyclooxygenase-2 inhibitors, and hospitalization for acute renal failure. They identified 121,722 new NSAID users older than age 65 years from the administrative health care databases of Quebec, Canada, in 1999–2002. Data for 4,228 cases and 84,540 controls matched on age and follow-up time were analyzed by using conditional logistic regression, adjusted for sex, age, health status, health care utilization measures, exposure to contrast agents, and nephrotoxic medications. The risk of acute renal failure for all NSAIDs combined was highest within 30 days of treatment initiation (adjusted rate ratio (RR) = 2.05, 95% confidence interval (CI): 1.61, 2.60) and receded thereafter. The association with acute renal failure within 30 days of therapy initiation was comparable for rofecoxib (RR = 2.31, 95% CI: 1.73, 3.08), naproxen (RR = 2.42, 95% CI: 1.52, 3.85), and nonselective, non-naproxen NSAIDs (RR = 2.30, 95% CI: 1.60, 3.32) but was borderline lower for celecoxib (RR =1.54, 95% CI: 1.14, 2.09; test for interaction comparing celecoxib with rofecoxib, p = 0.057). There was a significant association for both selective and nonselective NSAIDs with acute renal failure, but confirmatory studies are required.
The analgesic and antiinflammatory properties of nonsteroidal antiinflammatory drugs (NSAIDs) are based on inhibition of the cyclooxygenase (COX) enzyme isoforms. Because of a theoretically superior gastrointestinal safety profile, highly selective COX-2-inhibiting NSAIDs were developed (1). While recent attention has been focused on the cardiovascular risks of these new drugs, little attention has been paid to their possible renal toxicity. Additional information with respect to other critical adverse effects, such as nephrotoxicity, will allow a more comprehensive evaluation of the risks and benefits of the novel COX-2-inhibiting NSAIDs.
Conventional as well as COX-2-selective NSAIDs can cause renal complications, including rises in blood pressure, peripheral edema, sodium retention, and hyperkalemia (2–5). Although acute renal failure after exposure to conventional, nonselective NSAIDs has been shown in population-based studies (6–9), evaluation of the renal risk of COX-2 inhibitors is currently limited to sporadic case reports (10–14); thus, its full magnitude is unclear. Therefore, we analyzed a population-based cohort of new, elderly NSAID users to evaluate the time-dependent association of NSAID use, either nonselective or selective, with acute renal failure compared with that for individuals not exposed to these drugs.
MATERIALS AND METHODS
Data source
The databases of the universal health care program for residents of Quebec, Canada, older than 65 years of age were analyzed. These databases have been previously validated (15) and used for research (16–19).
We obtained information on demographics, duration of health care coverage, and all dispensed prescription drugs, including dose and duration. This information could be linked via an individual encrypted identifier, thereby maintaining confidentiality, to 1) the hospitalization database, which includes up to 15 International Classification of Diseases, Ninth Revision, diagnoses and seven treatment codes; 2) the physician services database; and 3) the vital statistics registry.
Cohort definition and follow-up
We conducted a nested case-control analysis (20). As reported previously (18), a population-based cohort of new NSAID users aged 66 years or older was formed, since universal drug coverage is available for only those residents older than age 65 years. Individuals were eligible for the study cohort if they had filled one or more prescriptions for an NSAID during the cohort entry period of January 1, 1999, to June 30, 2002, and were NSAID prescription free for at least 1 year before cohort entry. Data were restricted by the province's ethics board (Commission d'accès à l'information du Québec) to a random sample of 125,000 persons of the overall 302,964 fulfilling these criteria (18). We excluded individuals, whose only antiinflammatory during the study period was aspirin, who had a nonaspirin NSAID prescription in the year preceding cohort entry, who had been receiving renal replacement therapy (hemodialysis or peritoneal dialysis) in the year before cohort entry, had had kidney transplantation, or had received prescriptions for two different NSAIDs on the same date at cohort entry.
We defined cohort entry to be the date of the initial nonaspirin NSAID prescription, and individual follow-up started on this date. Follow-up ended on the index date, which was the date of the outcome (see below), the end of health insurance coverage, the date of the first dialysis procedure not fulfilling the outcome definition (e.g., for chronic renal failure), the date of renal transplantation, the date of death, or the end of the study on December 31, 2002, whichever occurred first.
Case definition
During follow-up, we identified as cases all cohort members with the a priori defined outcome, namely, a hospitalization containing a discharge diagnosis of acute renal failure (International Classification of Diseases, Ninth Revision, code 584) or, based on Perez Gutthann (7), unspecified renal failure (International Classification of Diseases, Ninth Revision, code 586). For each case, the date of admission of this hospitalization was the index date.
Control selection
For each case, we randomly selected up to 20 controls matched to cases on year and month of cohort entry as well as age at cohort entry (±1 year). Each control was assigned as index date the date of the outcome of his or her corresponding case, thereby assuring equal follow-up times.
Exposure categories and assessment of exposure
We a priori defined five mutually exclusive NSAID categories: 1) celecoxib (selective COX-2 inhibitor (21)); 2) rofecoxib (selective COX-2 inhibitor (21)); 3) meloxicam (selective COX-2 inhibitor (21)); 4) naproxen (nonselective COX-1 and COX-2 inhibitor (21), comparator in previous studies (22) and presumed favorable cardiovascular safety profile (23)); and 5) conventional NSAIDs (nonselective NSAIDs not contained in the previous four categories and excluding aspirin but listed in the provincial drug formulary during the study period (diclofenac, diflunisal, etodolac, fenoprofen, flurbiprofen, ibuprofen, indomethacin, ketoprofen, mefenamic acid, nabumetone, phenylbutazone, piroxicam, salsalate, sulindac, tenoxicam, tiaprofenic acid, and tolmetin). The first four NSAID categories were subdivided on the basis of average daily doses, as follows: 1) celecoxib: low dose ≤200 mg, high dose >200 mg; 2) rofecoxib: low dose ≤25 mg, high dose >25 mg; 3) meloxicam: low dose ≤7.5 mg, high dose >7.5 mg; and 4) naproxen: low dose ≤750 mg, high dose >750 mg.
Exposure to NSAIDs was assessed in the year preceding the index date. Based on the dispensing dates of the NSAID prescriptions, exposure was categorized with respect to the 1–30-, 31–90-, and 91–365-day periods preceding the index date. Considering all possible combinations of three periods, we created seven mutually exclusive exposure categories (table 1).
Definition of exposure-time categories for acute renal failure cases and controls based on NSAID* prescription(s) during the 365 days before the index date, Province of Quebec, Canada, 1999–2002
Exposure-time category . | Date of NSAID prescription(s)† . | . | . | ||
|---|---|---|---|---|---|
| . | 365–91 days‡ . | 90–31 days‡ . | 30–1 days‡ . | ||
| Current new use | − | − | + | ||
| Current and recent use | − | + | + | ||
| Current and past use | + | − | + | ||
| Current continuous use | + | + | + | ||
| Recent use | + or − | + | − | ||
| Past use | + | − | − | ||
| Unexposed | − | − | − | ||
Exposure-time category . | Date of NSAID prescription(s)† . | . | . | ||
|---|---|---|---|---|---|
| . | 365–91 days‡ . | 90–31 days‡ . | 30–1 days‡ . | ||
| Current new use | − | − | + | ||
| Current and recent use | − | + | + | ||
| Current and past use | + | − | + | ||
| Current continuous use | + | + | + | ||
| Recent use | + or − | + | − | ||
| Past use | + | − | − | ||
| Unexposed | − | − | − | ||
NSAID, nonsteroidal antiinflammatory drug.
+, presence of and –, absence of at least one prescription.
Before the index date.
Definition of exposure-time categories for acute renal failure cases and controls based on NSAID* prescription(s) during the 365 days before the index date, Province of Quebec, Canada, 1999–2002
Exposure-time category . | Date of NSAID prescription(s)† . | . | . | ||
|---|---|---|---|---|---|
| . | 365–91 days‡ . | 90–31 days‡ . | 30–1 days‡ . | ||
| Current new use | − | − | + | ||
| Current and recent use | − | + | + | ||
| Current and past use | + | − | + | ||
| Current continuous use | + | + | + | ||
| Recent use | + or − | + | − | ||
| Past use | + | − | − | ||
| Unexposed | − | − | − | ||
Exposure-time category . | Date of NSAID prescription(s)† . | . | . | ||
|---|---|---|---|---|---|
| . | 365–91 days‡ . | 90–31 days‡ . | 30–1 days‡ . | ||
| Current new use | − | − | + | ||
| Current and recent use | − | + | + | ||
| Current and past use | + | − | + | ||
| Current continuous use | + | + | + | ||
| Recent use | + or − | + | − | ||
| Past use | + | − | − | ||
| Unexposed | − | − | − | ||
NSAID, nonsteroidal antiinflammatory drug.
+, presence of and –, absence of at least one prescription.
Before the index date.
Statistical analysis and adjustment for potential confounders
The association between NSAIDs and acute renal failure was assessed by using multiple conditional logistic regression. We adjusted for the potentially confounding effects of sex, age, and numerous comorbid conditions, including preexisting renal disease and previous acute renal failure, assessed within the year preceding cohort entry by using hospitalization discharge codes and prescriptions (table 2).
Characteristics of acute renal failure cases and matched controls from a cohort of 121,722 new users of NSAIDs* older than 66 years of age from the Province of Quebec, Canada, 1999–2002
. | Cases (n = 4,228) . | Controls (n = 84,540) . | Adjusted RR*,† . | 95% CI* . |
|---|---|---|---|---|
| Age in years (mean (standard deviation))‡ | 78.1 (5.7) | 78.0 (5.7) | 1.09 | 1.03, 1.15 |
| Male sex (%) | 46.1 | 32.3 | 1.78 | 1.65, 1.90 |
| Female sex (%) | 53.9 | 67.7 | ||
| Comorbidity (%)§ | ||||
| Hypertension | 78.5 | 60.7 | 1.15 | 1.05, 1.26 |
| Diabetes | 26.4 | 11.5 | 1.16 | 1.06, 1.27 |
| Heart failure | 39.8 | 14.9 | 1.70 | 1.57, 1.85 |
| Cardiovascular disease | 42.0 | 21.2 | 1.13 | 1.04, 1.22 |
| Atherosclerosis | 7.7 | 2.3 | 1.08 | 0.93, 1.25 |
| Hyperlipidemia | 24.9 | 20.1 | 0.79 | 0.72, 0.85 |
| Respiratory disease | 33.0 | 19.5 | 0.86 | 0.79, 0.94 |
| Gastrointestinal ulcer disease | 37.6 | 28.2 | 0.87 | 0.81, 0.94 |
| Chronic renal failure | 4.8 | 0.7 | 1.49 | 1.22, 1.83 |
| Acute renal failure | 4.2 | 0.5 | 2.41 | 1.93, 3.01 |
| Renal disease | 1.1 | 0.2 | 0.94 | 0.64, 1.39 |
| Renovascular disease | 0.3 | 0.0 | 2.66 | 1.19, 5.96 |
| Renal infection | 3.5 | 1.4 | 1.05 | 0.86, 1.28 |
| Conditions secondary to renal impairment | 0.1 | 0.0 | 2.91 | 0.77, 10.97 |
| Renal manifestation of systemic diseases | 4.0 | 0.7 | 1.33 | 1.07, 1.65 |
| Systemic disease and malignancy relevant to renal function | 3.5 | 0.8 | 1.31 | 1.05, 1.63 |
| Drug use (%)§ | ||||
| Oral anticoagulants | 12.7 | 4.9 | 0.95 | 0.84, 1.06 |
| Oral corticosteroids | 13.7 | 6.6 | 0.96 | 0.86, 1.07 |
| Psychotropic drugs | 19.7 | 15.2 | 0.99 | 0.90, 1.08 |
| Thyroid drugs | 19.1 | 17.4 | 1.05 | 0.96, 1.14 |
| Current use of aspirin (%)¶ | 32.1 | 22.1 | 1.00 | 0.93, 1.08 |
| Use of nephrotoxic drugs (%)# | 73.6 | 49.3 | 1.55 | 1.43, 1.68 |
| Exposure to contrast media (%)# | 4.6 | 0.8 | 3.71 | 3.07, 4.49 |
| Comorbidity measures (mean (standard deviation))** | ||||
| No. of different drugs | 13.9 (6.7) | 8.6 (5.3) | 1.07 | 1.06, 1.08 |
| Chronic disease score | 8.1 (3.9) | 5.0 (3.5) | 1.05 | 1.03, 1.06 |
| Charlson index | 1.7 (2.5) | 0.4 (1.2) | 1.18 | 1.16, 1.20 |
| Health care utilization (%)** | ||||
| >12 physician visits | 59.6 | 38.5 | 1.05 | 0.98, 1.13 |
| ≥1 nephrologist visits | 5.5 | 1.7 | 1.71 | 1.45, 2.01 |
| >1 hospitalization | 27.8 | 9.0 | 1.14 | 1.03, 1.26 |
. | Cases (n = 4,228) . | Controls (n = 84,540) . | Adjusted RR*,† . | 95% CI* . |
|---|---|---|---|---|
| Age in years (mean (standard deviation))‡ | 78.1 (5.7) | 78.0 (5.7) | 1.09 | 1.03, 1.15 |
| Male sex (%) | 46.1 | 32.3 | 1.78 | 1.65, 1.90 |
| Female sex (%) | 53.9 | 67.7 | ||
| Comorbidity (%)§ | ||||
| Hypertension | 78.5 | 60.7 | 1.15 | 1.05, 1.26 |
| Diabetes | 26.4 | 11.5 | 1.16 | 1.06, 1.27 |
| Heart failure | 39.8 | 14.9 | 1.70 | 1.57, 1.85 |
| Cardiovascular disease | 42.0 | 21.2 | 1.13 | 1.04, 1.22 |
| Atherosclerosis | 7.7 | 2.3 | 1.08 | 0.93, 1.25 |
| Hyperlipidemia | 24.9 | 20.1 | 0.79 | 0.72, 0.85 |
| Respiratory disease | 33.0 | 19.5 | 0.86 | 0.79, 0.94 |
| Gastrointestinal ulcer disease | 37.6 | 28.2 | 0.87 | 0.81, 0.94 |
| Chronic renal failure | 4.8 | 0.7 | 1.49 | 1.22, 1.83 |
| Acute renal failure | 4.2 | 0.5 | 2.41 | 1.93, 3.01 |
| Renal disease | 1.1 | 0.2 | 0.94 | 0.64, 1.39 |
| Renovascular disease | 0.3 | 0.0 | 2.66 | 1.19, 5.96 |
| Renal infection | 3.5 | 1.4 | 1.05 | 0.86, 1.28 |
| Conditions secondary to renal impairment | 0.1 | 0.0 | 2.91 | 0.77, 10.97 |
| Renal manifestation of systemic diseases | 4.0 | 0.7 | 1.33 | 1.07, 1.65 |
| Systemic disease and malignancy relevant to renal function | 3.5 | 0.8 | 1.31 | 1.05, 1.63 |
| Drug use (%)§ | ||||
| Oral anticoagulants | 12.7 | 4.9 | 0.95 | 0.84, 1.06 |
| Oral corticosteroids | 13.7 | 6.6 | 0.96 | 0.86, 1.07 |
| Psychotropic drugs | 19.7 | 15.2 | 0.99 | 0.90, 1.08 |
| Thyroid drugs | 19.1 | 17.4 | 1.05 | 0.96, 1.14 |
| Current use of aspirin (%)¶ | 32.1 | 22.1 | 1.00 | 0.93, 1.08 |
| Use of nephrotoxic drugs (%)# | 73.6 | 49.3 | 1.55 | 1.43, 1.68 |
| Exposure to contrast media (%)# | 4.6 | 0.8 | 3.71 | 3.07, 4.49 |
| Comorbidity measures (mean (standard deviation))** | ||||
| No. of different drugs | 13.9 (6.7) | 8.6 (5.3) | 1.07 | 1.06, 1.08 |
| Chronic disease score | 8.1 (3.9) | 5.0 (3.5) | 1.05 | 1.03, 1.06 |
| Charlson index | 1.7 (2.5) | 0.4 (1.2) | 1.18 | 1.16, 1.20 |
| Health care utilization (%)** | ||||
| >12 physician visits | 59.6 | 38.5 | 1.05 | 0.98, 1.13 |
| ≥1 nephrologist visits | 5.5 | 1.7 | 1.71 | 1.45, 2.01 |
| >1 hospitalization | 27.8 | 9.0 | 1.14 | 1.03, 1.26 |
NSAIDs, nonsteroidal antiinflammatory drugs; RR, rate ratio; CI, confidence interval.
Adjusted for all other covariates listed in the table.
At cohort entry (date of first prescription of a nonaspirin nonsteroidal antiinflammatory drug).
Assessed in the year before cohort entry.
Overlap of last prescription with the index date.
Dichotomous (yes or no), assessed in the 30-day period before the index date.
Assessed in the year before the index date.
Characteristics of acute renal failure cases and matched controls from a cohort of 121,722 new users of NSAIDs* older than 66 years of age from the Province of Quebec, Canada, 1999–2002
. | Cases (n = 4,228) . | Controls (n = 84,540) . | Adjusted RR*,† . | 95% CI* . |
|---|---|---|---|---|
| Age in years (mean (standard deviation))‡ | 78.1 (5.7) | 78.0 (5.7) | 1.09 | 1.03, 1.15 |
| Male sex (%) | 46.1 | 32.3 | 1.78 | 1.65, 1.90 |
| Female sex (%) | 53.9 | 67.7 | ||
| Comorbidity (%)§ | ||||
| Hypertension | 78.5 | 60.7 | 1.15 | 1.05, 1.26 |
| Diabetes | 26.4 | 11.5 | 1.16 | 1.06, 1.27 |
| Heart failure | 39.8 | 14.9 | 1.70 | 1.57, 1.85 |
| Cardiovascular disease | 42.0 | 21.2 | 1.13 | 1.04, 1.22 |
| Atherosclerosis | 7.7 | 2.3 | 1.08 | 0.93, 1.25 |
| Hyperlipidemia | 24.9 | 20.1 | 0.79 | 0.72, 0.85 |
| Respiratory disease | 33.0 | 19.5 | 0.86 | 0.79, 0.94 |
| Gastrointestinal ulcer disease | 37.6 | 28.2 | 0.87 | 0.81, 0.94 |
| Chronic renal failure | 4.8 | 0.7 | 1.49 | 1.22, 1.83 |
| Acute renal failure | 4.2 | 0.5 | 2.41 | 1.93, 3.01 |
| Renal disease | 1.1 | 0.2 | 0.94 | 0.64, 1.39 |
| Renovascular disease | 0.3 | 0.0 | 2.66 | 1.19, 5.96 |
| Renal infection | 3.5 | 1.4 | 1.05 | 0.86, 1.28 |
| Conditions secondary to renal impairment | 0.1 | 0.0 | 2.91 | 0.77, 10.97 |
| Renal manifestation of systemic diseases | 4.0 | 0.7 | 1.33 | 1.07, 1.65 |
| Systemic disease and malignancy relevant to renal function | 3.5 | 0.8 | 1.31 | 1.05, 1.63 |
| Drug use (%)§ | ||||
| Oral anticoagulants | 12.7 | 4.9 | 0.95 | 0.84, 1.06 |
| Oral corticosteroids | 13.7 | 6.6 | 0.96 | 0.86, 1.07 |
| Psychotropic drugs | 19.7 | 15.2 | 0.99 | 0.90, 1.08 |
| Thyroid drugs | 19.1 | 17.4 | 1.05 | 0.96, 1.14 |
| Current use of aspirin (%)¶ | 32.1 | 22.1 | 1.00 | 0.93, 1.08 |
| Use of nephrotoxic drugs (%)# | 73.6 | 49.3 | 1.55 | 1.43, 1.68 |
| Exposure to contrast media (%)# | 4.6 | 0.8 | 3.71 | 3.07, 4.49 |
| Comorbidity measures (mean (standard deviation))** | ||||
| No. of different drugs | 13.9 (6.7) | 8.6 (5.3) | 1.07 | 1.06, 1.08 |
| Chronic disease score | 8.1 (3.9) | 5.0 (3.5) | 1.05 | 1.03, 1.06 |
| Charlson index | 1.7 (2.5) | 0.4 (1.2) | 1.18 | 1.16, 1.20 |
| Health care utilization (%)** | ||||
| >12 physician visits | 59.6 | 38.5 | 1.05 | 0.98, 1.13 |
| ≥1 nephrologist visits | 5.5 | 1.7 | 1.71 | 1.45, 2.01 |
| >1 hospitalization | 27.8 | 9.0 | 1.14 | 1.03, 1.26 |
. | Cases (n = 4,228) . | Controls (n = 84,540) . | Adjusted RR*,† . | 95% CI* . |
|---|---|---|---|---|
| Age in years (mean (standard deviation))‡ | 78.1 (5.7) | 78.0 (5.7) | 1.09 | 1.03, 1.15 |
| Male sex (%) | 46.1 | 32.3 | 1.78 | 1.65, 1.90 |
| Female sex (%) | 53.9 | 67.7 | ||
| Comorbidity (%)§ | ||||
| Hypertension | 78.5 | 60.7 | 1.15 | 1.05, 1.26 |
| Diabetes | 26.4 | 11.5 | 1.16 | 1.06, 1.27 |
| Heart failure | 39.8 | 14.9 | 1.70 | 1.57, 1.85 |
| Cardiovascular disease | 42.0 | 21.2 | 1.13 | 1.04, 1.22 |
| Atherosclerosis | 7.7 | 2.3 | 1.08 | 0.93, 1.25 |
| Hyperlipidemia | 24.9 | 20.1 | 0.79 | 0.72, 0.85 |
| Respiratory disease | 33.0 | 19.5 | 0.86 | 0.79, 0.94 |
| Gastrointestinal ulcer disease | 37.6 | 28.2 | 0.87 | 0.81, 0.94 |
| Chronic renal failure | 4.8 | 0.7 | 1.49 | 1.22, 1.83 |
| Acute renal failure | 4.2 | 0.5 | 2.41 | 1.93, 3.01 |
| Renal disease | 1.1 | 0.2 | 0.94 | 0.64, 1.39 |
| Renovascular disease | 0.3 | 0.0 | 2.66 | 1.19, 5.96 |
| Renal infection | 3.5 | 1.4 | 1.05 | 0.86, 1.28 |
| Conditions secondary to renal impairment | 0.1 | 0.0 | 2.91 | 0.77, 10.97 |
| Renal manifestation of systemic diseases | 4.0 | 0.7 | 1.33 | 1.07, 1.65 |
| Systemic disease and malignancy relevant to renal function | 3.5 | 0.8 | 1.31 | 1.05, 1.63 |
| Drug use (%)§ | ||||
| Oral anticoagulants | 12.7 | 4.9 | 0.95 | 0.84, 1.06 |
| Oral corticosteroids | 13.7 | 6.6 | 0.96 | 0.86, 1.07 |
| Psychotropic drugs | 19.7 | 15.2 | 0.99 | 0.90, 1.08 |
| Thyroid drugs | 19.1 | 17.4 | 1.05 | 0.96, 1.14 |
| Current use of aspirin (%)¶ | 32.1 | 22.1 | 1.00 | 0.93, 1.08 |
| Use of nephrotoxic drugs (%)# | 73.6 | 49.3 | 1.55 | 1.43, 1.68 |
| Exposure to contrast media (%)# | 4.6 | 0.8 | 3.71 | 3.07, 4.49 |
| Comorbidity measures (mean (standard deviation))** | ||||
| No. of different drugs | 13.9 (6.7) | 8.6 (5.3) | 1.07 | 1.06, 1.08 |
| Chronic disease score | 8.1 (3.9) | 5.0 (3.5) | 1.05 | 1.03, 1.06 |
| Charlson index | 1.7 (2.5) | 0.4 (1.2) | 1.18 | 1.16, 1.20 |
| Health care utilization (%)** | ||||
| >12 physician visits | 59.6 | 38.5 | 1.05 | 0.98, 1.13 |
| ≥1 nephrologist visits | 5.5 | 1.7 | 1.71 | 1.45, 2.01 |
| >1 hospitalization | 27.8 | 9.0 | 1.14 | 1.03, 1.26 |
NSAIDs, nonsteroidal antiinflammatory drugs; RR, rate ratio; CI, confidence interval.
Adjusted for all other covariates listed in the table.
At cohort entry (date of first prescription of a nonaspirin nonsteroidal antiinflammatory drug).
Assessed in the year before cohort entry.
Overlap of last prescription with the index date.
Dichotomous (yes or no), assessed in the 30-day period before the index date.
Assessed in the year before the index date.
Preexisting renal disease was further subclassified into chronic renal failure, previous acute renal failure, renal disease (nephrotic syndrome, nephritis, renal sclerosis, cystic kidney disease, reflux), renovascular disease (sclerosis and aneurysm of renal artery), renal infection (pyelonephritis, renal abscess, urinary tract infection), conditions secondary to renal impairment (renal osteodystrophy, nephrogenic diabetes insipidus, secondary hypertension), renal manifestation of systemic diseases (hypertensive and diabetic renal disease, gouty nephropathy, hepatorenal syndrome), or systemic diseases and malignancy relevant to renal function (neoplasm of urinary tract and kidney, leukemia, myeloma, metastatic cancer, disorders of plasma protein metabolism, connective tissue disease, polyarteritis).
We also considered as potential confounders exposure to nephrotoxic drugs (diuretics, antibiotics, drugs acting on the nervous system, immunosuppressants, and others (24, 25)) and contrast media (procedure codes indicating intravenous application) during the 30 days preceding the index date. Moreover, we assessed prescriptions for anticoagulants, corticosteroids, psychotropics, and thyroid drugs in the year before cohort entry. We determined the number of distinct drugs prescribed (26), a chronic disease score (27), the Charlson index (28), and measures of health care utilization (number of hospitalizations, outpatient physician and nephrologist encounters) in the year before the index date. As reported in previous investigations (6–8), we considered aspirin separately. We defined exposure to aspirin as overlapping of the last prescription with the index date.
We assessed the stability of the incidence rate of acute renal failure by dividing the follow-up time into 16 equal intervals and tested for significance by using a Poisson regression model (29).
Drug switching and concurrent prescription of more than one NSAID led to multiple different exposure patterns; therefore, stratification of the specific drug exposure categories other than for current new use was perceived to be inefficient. Consequently, to study the independent effects of the various NSAID categories, we analyzed individual NSAID categories for current new users only because doing so allowed for the most meaningful and unequivocal interpretation. Finally, we considered the average daily dose of the NSAID in current new users, adjusting for all potential confounders and comparing with individuals unexposed to NSAIDs in the year before their index date.
SAS version 8.2 software (SAS Institute, Inc., Cary, North Carolina) was used for data analysis.
The study was approved by the Commission d'Accès à l'Information du Québec and the Institutional Review Board of the Faculty of Medicine, McGill University.
RESULTS
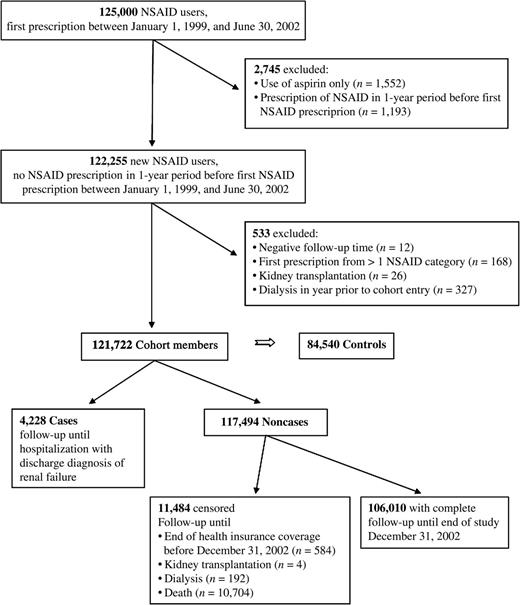
A cohort of 121,722 new users of NSAIDs (figure 1) was followed up for an average of 2.37 years (standard deviation, 0.97). Overall, 288,364 person-years of observation were available for analysis. During this time, we identified 4,228 cases of acute renal failure (International Classification of Diseases, Ninth Revision, codes 584 and 586 in 73.7 percent and 26.3 percent of cases, respectively) and 84,540 matched controls. The average incidence rate of acute renal failure (1.48 cases/100 person-years; standard deviation, 0.34) was stable throughout the study period (χ2 test, p = 0.43). Case fatality during follow-up was high (47.3 percent), with a median survival of 35 days (quartile 1: 10 days, quartile 3: 156 days) after the diagnosis of acute renal failure.
Flow of study participants older than 66 years of age from the Province of Quebec, Canada, 1999–2002. Controls for each case were selected from cohort members still free of the outcome and not yet censored at the case's index date (incidence density sampling). NSAID, nonsteroidal antiinflammatory drug, excluding aspirin.
Cases were more likely to be male and to have hypertension, diabetes, and preexisting renal diseases, including previous episodes of acute renal failure (table 2). In the year before the index date, cases, compared with controls, used more health care services and, on average, required a higher number of drugs. Exposure to nephrotoxic drugs and contrast media was also more frequent in cases. The median duration of NSAID prescriptions was 30 days (quartile 1: 12 days, quartile 3: 30 days).
Overall, current new users of any NSAID, compared with unexposed individuals, were at highest risk of acute renal failure (table 3; adjusted rate ratio (RR) = 2.05, 95 percent confidence interval (CI): 1.61, 2.60). Current and recent users appeared to have a slightly lower risk (RR = 1.62, 95 percent CI: 1.29, 2.04). This risk decreased further with increasing duration of use (RR for current continuous users = 1.14, 95 percent CI: 1.01, 1.28). The risk for those who were reexposed to an NSAID (current and past users) was intermediate (RR = 1.26, 95 percent CI: 1.04, 1.53). For individuals who had recently stopped using NSAIDs (recent users), the risk of acute renal failure was indistinguishable from that of the reference category (RR = 0.96, 95 percent CI: 0.85, 1.08). Past users had a lower risk than unexposed individuals (RR = 0.77, 95 percent CI: 0.69, 0.85).
Rates of acute renal failure for NSAID* users older than 66 years of age from the Province of Quebec, Canada, 1999–2002, dependent on time, all NSAID categories combined, relative to rates for individuals without an NSAID prescription for 365 days
Exposure-time category† . | No. of cases (n = 4,228) . | No. of controls (n = 84,540) . | Unadjusted RR* . | 95% CI* . | Adjusted RR‡ . | 95% CI . |
|---|---|---|---|---|---|---|
| Unexposed (reference category) | 1,130 | 24,566 | 1.00 | 1.00 | ||
| Current new use | 382 | 6,576 | 2.31 | 1.85, 2.87 | 2.05 | 1.61, 2.60 |
| Current and recent use | 149 | 2,205 | 1.83 | 1.47, 2.26 | 1.62 | 1.29, 2.04 |
| Current and past use | 153 | 2,181 | 1.53 | 1.28, 1.82 | 1.26 | 1.04, 1.53 |
| Current continuous use | 602 | 8,833 | 1.49 | 1.34, 1.66 | 1.14 | 1.01, 1.28 |
| Recent use | 630 | 13,200 | 1.13 | 1.00, 1.26 | 0.96 | 0.85, 1.08 |
| Past use | 1,182 | 26,979 | 0.95 | 0.86, 1.04 | 0.77 | 0.69, 0.85 |
Exposure-time category† . | No. of cases (n = 4,228) . | No. of controls (n = 84,540) . | Unadjusted RR* . | 95% CI* . | Adjusted RR‡ . | 95% CI . |
|---|---|---|---|---|---|---|
| Unexposed (reference category) | 1,130 | 24,566 | 1.00 | 1.00 | ||
| Current new use | 382 | 6,576 | 2.31 | 1.85, 2.87 | 2.05 | 1.61, 2.60 |
| Current and recent use | 149 | 2,205 | 1.83 | 1.47, 2.26 | 1.62 | 1.29, 2.04 |
| Current and past use | 153 | 2,181 | 1.53 | 1.28, 1.82 | 1.26 | 1.04, 1.53 |
| Current continuous use | 602 | 8,833 | 1.49 | 1.34, 1.66 | 1.14 | 1.01, 1.28 |
| Recent use | 630 | 13,200 | 1.13 | 1.00, 1.26 | 0.96 | 0.85, 1.08 |
| Past use | 1,182 | 26,979 | 0.95 | 0.86, 1.04 | 0.77 | 0.69, 0.85 |
Rates of acute renal failure for NSAID* users older than 66 years of age from the Province of Quebec, Canada, 1999–2002, dependent on time, all NSAID categories combined, relative to rates for individuals without an NSAID prescription for 365 days
Exposure-time category† . | No. of cases (n = 4,228) . | No. of controls (n = 84,540) . | Unadjusted RR* . | 95% CI* . | Adjusted RR‡ . | 95% CI . |
|---|---|---|---|---|---|---|
| Unexposed (reference category) | 1,130 | 24,566 | 1.00 | 1.00 | ||
| Current new use | 382 | 6,576 | 2.31 | 1.85, 2.87 | 2.05 | 1.61, 2.60 |
| Current and recent use | 149 | 2,205 | 1.83 | 1.47, 2.26 | 1.62 | 1.29, 2.04 |
| Current and past use | 153 | 2,181 | 1.53 | 1.28, 1.82 | 1.26 | 1.04, 1.53 |
| Current continuous use | 602 | 8,833 | 1.49 | 1.34, 1.66 | 1.14 | 1.01, 1.28 |
| Recent use | 630 | 13,200 | 1.13 | 1.00, 1.26 | 0.96 | 0.85, 1.08 |
| Past use | 1,182 | 26,979 | 0.95 | 0.86, 1.04 | 0.77 | 0.69, 0.85 |
Exposure-time category† . | No. of cases (n = 4,228) . | No. of controls (n = 84,540) . | Unadjusted RR* . | 95% CI* . | Adjusted RR‡ . | 95% CI . |
|---|---|---|---|---|---|---|
| Unexposed (reference category) | 1,130 | 24,566 | 1.00 | 1.00 | ||
| Current new use | 382 | 6,576 | 2.31 | 1.85, 2.87 | 2.05 | 1.61, 2.60 |
| Current and recent use | 149 | 2,205 | 1.83 | 1.47, 2.26 | 1.62 | 1.29, 2.04 |
| Current and past use | 153 | 2,181 | 1.53 | 1.28, 1.82 | 1.26 | 1.04, 1.53 |
| Current continuous use | 602 | 8,833 | 1.49 | 1.34, 1.66 | 1.14 | 1.01, 1.28 |
| Recent use | 630 | 13,200 | 1.13 | 1.00, 1.26 | 0.96 | 0.85, 1.08 |
| Past use | 1,182 | 26,979 | 0.95 | 0.86, 1.04 | 0.77 | 0.69, 0.85 |
We also examined the risks for current new users of specific NSAIDs. Compared with unexposed individuals, current new users of conventional NSAIDs (RR = 2.30, 95 percent CI: 1.60, 3.32), rofecoxib (RR = 2.31, 95 percent CI: 1.73, 3.08), and naproxen (RR = 2.42, 95 percent CI: 1.52, 3.85) had higher risks of acute renal failure (table 4). In contrast, the risk of renal failure for current new users of celecoxib was lower than that for the other NSAIDs although increased compared with those unexposed (RR = 1.54, 95 percent CI: 1.14, 2.09). No difference between the risks for current new users of rofecoxib and celecoxib was found when assessed with an interaction test (30) (two-sided p = 0.057). There were too few current new users of meloxicam to reliably determine the risk of acute renal failure (RR = 1.27, 95 percent CI: 0.36, 4.45). Notably, acute renal failure was strongly associated with use of agents from more than one NSAID category during the 30 days preceding the index date (RR = 4.65, 95 percent CI: 2.31, 9.37). In 61.9 percent of these individuals, the dates of the prescriptions from the two different NSAID categories overlapped.
Rates of acute renal failure by NSAID* category for current new NSAID users older than 66 years of age from the Province of Quebec, Canada, 1999–2002, relative to rates for individuals without an NSAID prescription for 365 days
Exposure-time category† . | No. of cases . | No. of controls . | Unadjusted RR* . | 95% CI* . | Adjusted RR‡ . | 95% CI . |
|---|---|---|---|---|---|---|
| Unexposed (reference category) | 1,130 | 24,566 | 1.00 | 1.00 | ||
| Current new use | ||||||
| Conventional NSAIDs | 75 | 1,252 | 2.54 | 1.82, 3.55 | 2.30 | 1.60, 3.32 |
| Rofecoxib | 145 | 2,217 | 2.58 | 1.98, 3.36 | 2.31 | 1.73, 3.08 |
| Celecoxib | 112 | 2,456 | 1.73 | 1.30, 2.29 | 1.54 | 1.14, 2.09 |
| Naproxen | 35 | 478 | 3.12 | 2.05, 4.74 | 2.42 | 1.52, 3.85 |
| Meloxicam | 3 | 72 | 1.47 | 0.46, 4.73 | 1.27 | 0.36, 4.45 |
| NSAIDs from >1 category | 12 | 101 | 4.45 | 2.38, 8.33 | 4.65 | 2.31, 9.37 |
Exposure-time category† . | No. of cases . | No. of controls . | Unadjusted RR* . | 95% CI* . | Adjusted RR‡ . | 95% CI . |
|---|---|---|---|---|---|---|
| Unexposed (reference category) | 1,130 | 24,566 | 1.00 | 1.00 | ||
| Current new use | ||||||
| Conventional NSAIDs | 75 | 1,252 | 2.54 | 1.82, 3.55 | 2.30 | 1.60, 3.32 |
| Rofecoxib | 145 | 2,217 | 2.58 | 1.98, 3.36 | 2.31 | 1.73, 3.08 |
| Celecoxib | 112 | 2,456 | 1.73 | 1.30, 2.29 | 1.54 | 1.14, 2.09 |
| Naproxen | 35 | 478 | 3.12 | 2.05, 4.74 | 2.42 | 1.52, 3.85 |
| Meloxicam | 3 | 72 | 1.47 | 0.46, 4.73 | 1.27 | 0.36, 4.45 |
| NSAIDs from >1 category | 12 | 101 | 4.45 | 2.38, 8.33 | 4.65 | 2.31, 9.37 |
Rates of acute renal failure by NSAID* category for current new NSAID users older than 66 years of age from the Province of Quebec, Canada, 1999–2002, relative to rates for individuals without an NSAID prescription for 365 days
Exposure-time category† . | No. of cases . | No. of controls . | Unadjusted RR* . | 95% CI* . | Adjusted RR‡ . | 95% CI . |
|---|---|---|---|---|---|---|
| Unexposed (reference category) | 1,130 | 24,566 | 1.00 | 1.00 | ||
| Current new use | ||||||
| Conventional NSAIDs | 75 | 1,252 | 2.54 | 1.82, 3.55 | 2.30 | 1.60, 3.32 |
| Rofecoxib | 145 | 2,217 | 2.58 | 1.98, 3.36 | 2.31 | 1.73, 3.08 |
| Celecoxib | 112 | 2,456 | 1.73 | 1.30, 2.29 | 1.54 | 1.14, 2.09 |
| Naproxen | 35 | 478 | 3.12 | 2.05, 4.74 | 2.42 | 1.52, 3.85 |
| Meloxicam | 3 | 72 | 1.47 | 0.46, 4.73 | 1.27 | 0.36, 4.45 |
| NSAIDs from >1 category | 12 | 101 | 4.45 | 2.38, 8.33 | 4.65 | 2.31, 9.37 |
Exposure-time category† . | No. of cases . | No. of controls . | Unadjusted RR* . | 95% CI* . | Adjusted RR‡ . | 95% CI . |
|---|---|---|---|---|---|---|
| Unexposed (reference category) | 1,130 | 24,566 | 1.00 | 1.00 | ||
| Current new use | ||||||
| Conventional NSAIDs | 75 | 1,252 | 2.54 | 1.82, 3.55 | 2.30 | 1.60, 3.32 |
| Rofecoxib | 145 | 2,217 | 2.58 | 1.98, 3.36 | 2.31 | 1.73, 3.08 |
| Celecoxib | 112 | 2,456 | 1.73 | 1.30, 2.29 | 1.54 | 1.14, 2.09 |
| Naproxen | 35 | 478 | 3.12 | 2.05, 4.74 | 2.42 | 1.52, 3.85 |
| Meloxicam | 3 | 72 | 1.47 | 0.46, 4.73 | 1.27 | 0.36, 4.45 |
| NSAIDs from >1 category | 12 | 101 | 4.45 | 2.38, 8.33 | 4.65 | 2.31, 9.37 |
In all comparisons related to dose, current new use of higher doses of NSAIDs was associated with a higher risk of acute renal failure than for individuals unexposed to NSAIDs (table 5). For rofecoxib, the adjusted rate ratio for >25 mg/day was 6.64 (95 percent CI: 4.06, 10.87); for ≤25 mg/day, the rate ratio was 1.94 (95 percent CI: 1.44, 2.63; p < 0.001 for trend). The risks of acute renal failure in current new users of celecoxib were 2.00 (95 percent CI: 1.32, 3.04) for >200 mg/day and 1.33 (95 percent CI: 0.94, 1.88; p = 0.21 for trend) for ≤200 mg/day. Similarly, the adjusted rate ratios for >750 mg/day of naproxen were 3.62 (95 percent CI: 2.01, 6.53) and 1.65 (95 percent CI: 0.88, 3.08; p = 0.07 for trend) for ≤750 mg/day. There were insufficient current new users of meloxicam to assess a dose-response relation.
Rates of acute renal failure by dose for current new users of rofecoxib, celecoxib, and naproxen older than 66 years of age from the Province of Quebec, Canada, 1999–2002, relative to rates for individuals without an NSAID* prescription for 365 days
Exposure-time category† . | No. of cases . | No. of controls . | Unadjusted RR* . | 95% CI* . | Adjusted RR‡ . | 95% CI . |
|---|---|---|---|---|---|---|
| Unexposed (reference category) | 1,130 | 24,566 | 1.00 | 1.00 | ||
| Current new use | ||||||
| Rofecoxib (mg/day)§ | ||||||
| High dose (>25) | 28 | 189 | 5.75 | 3.68, 8.99 | 6.64 | 4.06, 10.87 |
| Low dose (≤25) | 117 | 2,028 | 2.25 | 1.71, 2.97 | 1.94 | 1.44, 2.63 |
| Celecoxib (mg/day)¶ | ||||||
| High dose (>200) | 39 | 553 | 2.57 | 1.76, 3.76 | 2.00 | 1.32, 3.04 |
| Low dose (≤200) | 73 | 1,903 | 1.42 | 1.03, 1.95 | 1.33 | 0.94, 1.88 |
| Naproxen (mg/day)# | ||||||
| High dose (>750) | 19 | 165 | 4.75 | 2.79, 8.07 | 3.62 | 2.01, 6.53 |
| Low dose (≤750) | 16 | 313 | 2.16 | 1.23, 3.78 | 1.65 | 0.88, 3.08 |
Exposure-time category† . | No. of cases . | No. of controls . | Unadjusted RR* . | 95% CI* . | Adjusted RR‡ . | 95% CI . |
|---|---|---|---|---|---|---|
| Unexposed (reference category) | 1,130 | 24,566 | 1.00 | 1.00 | ||
| Current new use | ||||||
| Rofecoxib (mg/day)§ | ||||||
| High dose (>25) | 28 | 189 | 5.75 | 3.68, 8.99 | 6.64 | 4.06, 10.87 |
| Low dose (≤25) | 117 | 2,028 | 2.25 | 1.71, 2.97 | 1.94 | 1.44, 2.63 |
| Celecoxib (mg/day)¶ | ||||||
| High dose (>200) | 39 | 553 | 2.57 | 1.76, 3.76 | 2.00 | 1.32, 3.04 |
| Low dose (≤200) | 73 | 1,903 | 1.42 | 1.03, 1.95 | 1.33 | 0.94, 1.88 |
| Naproxen (mg/day)# | ||||||
| High dose (>750) | 19 | 165 | 4.75 | 2.79, 8.07 | 3.62 | 2.01, 6.53 |
| Low dose (≤750) | 16 | 313 | 2.16 | 1.23, 3.78 | 1.65 | 0.88, 3.08 |
NSAID, nonsteroidal antiinflammatory drug; RR, rate ratio; CI, confidence interval.
Exposure-time categories are defined in table 1; results for the remaining NSAID categories and exposure-time categories are shown in table 4 and table 3, respectively.
Adjusted for all remaining NSAID categories listed in table 4, all exposure-time categories except for current new use listed in table 3, and all covariates listed in table 2.
p < 0.001 for trend.
p = 0.21 for trend.
p = 0.07 for trend.
Rates of acute renal failure by dose for current new users of rofecoxib, celecoxib, and naproxen older than 66 years of age from the Province of Quebec, Canada, 1999–2002, relative to rates for individuals without an NSAID* prescription for 365 days
Exposure-time category† . | No. of cases . | No. of controls . | Unadjusted RR* . | 95% CI* . | Adjusted RR‡ . | 95% CI . |
|---|---|---|---|---|---|---|
| Unexposed (reference category) | 1,130 | 24,566 | 1.00 | 1.00 | ||
| Current new use | ||||||
| Rofecoxib (mg/day)§ | ||||||
| High dose (>25) | 28 | 189 | 5.75 | 3.68, 8.99 | 6.64 | 4.06, 10.87 |
| Low dose (≤25) | 117 | 2,028 | 2.25 | 1.71, 2.97 | 1.94 | 1.44, 2.63 |
| Celecoxib (mg/day)¶ | ||||||
| High dose (>200) | 39 | 553 | 2.57 | 1.76, 3.76 | 2.00 | 1.32, 3.04 |
| Low dose (≤200) | 73 | 1,903 | 1.42 | 1.03, 1.95 | 1.33 | 0.94, 1.88 |
| Naproxen (mg/day)# | ||||||
| High dose (>750) | 19 | 165 | 4.75 | 2.79, 8.07 | 3.62 | 2.01, 6.53 |
| Low dose (≤750) | 16 | 313 | 2.16 | 1.23, 3.78 | 1.65 | 0.88, 3.08 |
Exposure-time category† . | No. of cases . | No. of controls . | Unadjusted RR* . | 95% CI* . | Adjusted RR‡ . | 95% CI . |
|---|---|---|---|---|---|---|
| Unexposed (reference category) | 1,130 | 24,566 | 1.00 | 1.00 | ||
| Current new use | ||||||
| Rofecoxib (mg/day)§ | ||||||
| High dose (>25) | 28 | 189 | 5.75 | 3.68, 8.99 | 6.64 | 4.06, 10.87 |
| Low dose (≤25) | 117 | 2,028 | 2.25 | 1.71, 2.97 | 1.94 | 1.44, 2.63 |
| Celecoxib (mg/day)¶ | ||||||
| High dose (>200) | 39 | 553 | 2.57 | 1.76, 3.76 | 2.00 | 1.32, 3.04 |
| Low dose (≤200) | 73 | 1,903 | 1.42 | 1.03, 1.95 | 1.33 | 0.94, 1.88 |
| Naproxen (mg/day)# | ||||||
| High dose (>750) | 19 | 165 | 4.75 | 2.79, 8.07 | 3.62 | 2.01, 6.53 |
| Low dose (≤750) | 16 | 313 | 2.16 | 1.23, 3.78 | 1.65 | 0.88, 3.08 |
NSAID, nonsteroidal antiinflammatory drug; RR, rate ratio; CI, confidence interval.
Exposure-time categories are defined in table 1; results for the remaining NSAID categories and exposure-time categories are shown in table 4 and table 3, respectively.
Adjusted for all remaining NSAID categories listed in table 4, all exposure-time categories except for current new use listed in table 3, and all covariates listed in table 2.
p < 0.001 for trend.
p = 0.21 for trend.
p = 0.07 for trend.
DISCUSSION
This is the first known population-based study to compare the association of both selective and nonselective NSAIDs with the risk of acute renal failure. We demonstrated that, within 1 month of a first prescription, there exists a twofold increase in the risk of acute renal failure with any NSAID and a fourfold increase with use of NSAIDs from more than one category, reflecting either early drug switching or concurrent use of more than one NSAID. Longer-term, continuous NSAID users remain at an increased but lower level of risk. After at least 30 days without an NSAID prescription, the risk of renal failure returned to baseline. Past users were at lower risk than unexposed individuals, possibly representing a selected population resistant to nephrotoxicity because of depletion of susceptible cases (31). Because the number of cases exposed to individual conventional NSAIDs was limited, it was impossible to provide a detailed evaluation of the known heterogeneous renal risk (8) of individual conventional NSAIDs other than naproxen. Small numbers also precluded an evaluation of the selective COX-2 inhibitor meloxicam.
To our knowledge, this is also the first study to consider dosages of specific COX-2-selective NSAIDs. Globally, low-dose rofecoxib, high-dose celecoxib, and nonselective NSAIDs appeared to be associated with comparable rates of acute renal failure. High-dose rofecoxib seemed to be the most, and low-dose celecoxib the least, nephrotoxic. Compared with the other NSAIDs, celecoxib tended to have a better renal safety profile, particularly at a dose of 200 mg/day or less.
Our observations of the overall risk of renal failure associated with rofecoxib are generally consistent with previous predictions (32–34). The apparently more favorable renal safety profile of celecoxib is in accordance with findings from the CLASS study (35), in which edema, hypertension, and increased creatinine levels occurred more often in the ibuprofen and diclofenac than in the celecoxib group. More patients using rofecoxib than using celecoxib developed edema and hypertension in the comparative SUCCESS study (36). Moreover, an analysis of the World Health Organization drug safety database also revealed that rofecoxib led to more renal-adverse events than did celecoxib (13). These findings do not support the assumption that the magnitude of the renal risk of NSAIDs can be predicted simply on the basis of the relative strength of COX-1/COX-2 selectivity.
We provided some evidence for a class effect for all NSAIDs, regardless of their COX-2 selectivity, with respect to the risk of acute renal failure. Nonetheless, there are important differences in the magnitude of the risk between these agents, most notably between celecoxib and the others. The pattern of risk we observed in our study is also in accordance with the variations in cardiovascular safety observed for selective COX-2 inhibitors (18, 37–39). Finally, the differences in risk between celecoxib and rofecoxib suggest that mechanisms other than inhibition of COX might be involved in the observed nephrotoxic effects of NSAIDs.
Although these results were derived by using an observational design, our study does have a number of strengths. Our analysis of a population-based cohort of incident users minimizes the potential for bias compared with other designs (40). Moreover, we carefully controlled for age, length of follow-up, and most known confounders, including preexisting renal disease and exposure to other nephrotoxic drugs. Our results are based on a large number of nonselected subjects and show a surprising high number of cases of acute renal failure, highlighting the clinical importance of the renal safety of NSAIDs in the elderly. Indeed, the incidence of acute renal failure was 50 percent higher than that of acute myocardial infarction in the same cohort (18).
Furthermore, the validity of our study is reinforced by the consistency of our results with previous knowledge. The risk of nonselective, NSAID-induced acute renal failure is known to be both time (8) and dose (7, 8) dependent. We also demonstrated the expected strong relation between exposure to contrast agents or nephrotoxic drugs or multiple NSAID exposure and acute renal failure.
Our study does have several limitations. We had no measure of the severity of renal impairment, such as serum creatinine values. However, this shortcoming did not affect the validity of our study addressing the risk of hospitalization for renal failure rather than the severity of the decline in renal function. Exposure to a conventional, nonselective NSAID might have caused physicians to more readily investigate for an adverse renal event (detection bias) because the association of NSAIDs with acute renal failure was well established previously, in contrast to the largely unstudied selective COX-2 inhibitors. However, our observation of similar risks for rofecoxib and conventional NSAIDs, as well as a differential risk across COX-2 inhibitors, indicates that this is unlikely to represent an important source of bias in our study. Our study could not distinguish between acute renal failure occurring before or during hospitalization. We cannot rule out the possibility that some cases represented in-hospital renal failure, and a previous investigation suggests that they account for approximately 11 percent of potential cases (7). However, if comorbidity rather than NSAID exposure is the key determinant of in-hospital renal failure, then misclassification of in-hospital cases as NSAID-associated cases is likely nondifferential with respect to NSAID exposure and would have led to underestimation of the true risks.
Individuals at increased risk of acute renal failure might have in fact been preferentially exposed to certain NSAIDs, leading to confounding by indication. It has been shown that patients with more severe comorbidity are preferentially prescribed COX-2 inhibitors compared with conventional NSAIDs (41). However, this factor would not explain the difference between the risks of individual COX-2 inhibitors observed here. Although the choice of NSAID appears to be influenced more strongly by physician characteristics than by patient comorbidity (42), we cannot exclude the possibility of residual confounding by indication.
Ibuprofen and aspirin are available over the counter in Canada, but they are also covered by the prescription drug plan for persons 65 years of age or older. Therefore, the proportion of over-the-counter NSAIDs users is expected to be small. Fortunately, any resulting misclassification of NSAID exposure, for example, due to short-term self-medication for acute pain, is expected to be nondifferential with respect to the NSAID categories in our study. Consequently, it would bias the results toward the null. Finally, important health determinants, such as smoking, obesity, and socioeconomic status, could not be considered in our investigation, but these conditions seem unlikely to be differentially related to NSAID exposure. Further studies are required to confirm our observations and to firmly delineate the renal toxicity of the novel NSAIDs. For example, might low-dose celecoxib represent an appropriate option for patients in whom maintenance of renal function is a priority?
Finally, the impact of use of NSAIDs on the overall caseload of renal failure due to any cause can be assessed by the population attributable fraction, which was 4.6 percent (95 percent CI: 3.1 percent, 6.1 percent) for current new users of NSAIDs. In absolute terms, this value implies that 196 patients in our cohort experienced acute renal failure due to recent initiation of NSAID treatment.
In conclusion, we determined that the effect of NSAIDs on kidney function appears to be acute and to recede over time. The risk of acute renal failure with low-dose rofecoxib is comparable to that of conventional NSAIDs, whereas it seems to be higher at daily doses of more than 25 mg. For celecoxib, the risk also appears to be dose dependent but weaker compared with both rofecoxib and conventional NSAIDs, although this observation requires confirmation in other studies. In assessing COX-2-inhibitor safety, one should not exclusively consider cardiovascular side effects but rather concentrate on overall risk profile, including the propensity for prognostically important renal dysfunction. The high risk of acute renal failure observed here reinforces the wisdom of maintaining the voluntary ban on rofecoxib initially mandated by cardiovascular safety concerns. The frequent occurrence of acute renal failure observed in our study means that future trials of NSAIDs should a priori diligently monitor renal function in a manner similar to that proposed for cardiovascular outcomes. However, given the highly selective nature of the study populations and the small sample sizes in most randomized controlled trials, population-based observational studies will continue to have an important role in the assessment of infrequent adverse effects.
This study was funded by a grant from the Canadian Institutes of Health Research (CIHR grant MOP62871).
Dr. Brophy is a Physician-Scientist of the Fonds de la recherche en santé du Québec. Dr. Schneider received a scholarship from the Research Institute of McGill University Health Center and the Department of Medicine of McGill University.
Conflict of interest: none declared.
References
FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2.
Clive DM, Stoff JS. Renal syndromes associated with nonsteroidal antiinflammatory drugs.
Whelton A, Hamilton CW. Nonsteroidal anti-inflammatory drugs: effects on kidney function.
Harris RC, Breyer MD. Arachidonic acid metabolites and the kidney. In: Brenner BM, ed. Brenner & Rector's the kidney. 7th ed. St. Louis, MO: W B Saunders,
Evans JM, McGregor E, McMahon AD, et al. Non-steroidal anti-inflammatory drugs and hospitalization for acute renal failure.
Perez Gutthann S, Garcia Rodriguez LA, Raiford DS, et al. Nonsteroidal anti-inflammatory drugs and the risk of hospitalization for acute renal failure.
Griffin MR, Yared A, Ray WA. Nonsteroidal antiinflammatory drugs and acute renal failure in elderly persons.
Huerta C, Castellsague J, Varas-Lorenzo C, et al. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population.
Layton D, Riley J, Wilton LV, et al. Safety profile of rofecoxib as used in general practice in England: results of a prescription-event monitoring study.
Ahmad SR, Kortepeter C, Brinker A, et al. Renal failure associated with the use of celecoxib and rofecoxib.
Perazella MA, Tray K. Selective cyclooxygenase-2 inhibitors: a pattern of nephrotoxicity similar to traditional nonsteroidal anti-inflammatory drugs.
Zhao SZ, Reynolds MW, Lejkowith J, et al. A comparison of renal-related adverse drug reactions between rofecoxib and celecoxib, based on the World Health Organization/Uppsala Monitoring Centre safety database.
Perazella MA, Eras J. Are selective COX-2 inhibitors nephrotoxic?
Tamblyn R, Lavoie G, Petrella L, et al. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Quebec.
Bardou M, Barkun AN, Ghosn J, et al. Effect of chronic intake of NSAIDs and cyclooxygenase 2-selective inhibitors on esophageal cancer incidence.
Quach C, Collet JP, LeLorier J. Effectiveness of amoxicillin, azithromycin, cefprozil and clarithromycin in the treatment of acute otitis media in children: a population-based study.
Levesque LE, Brophy JM, Zhang B. The risk for myocardial infarction with cyclooxygenase-2 inhibitors: a population study of elderly adults.
Garbe E, Suissa S, LeLorier J. Association of inhaled corticosteroid use with cataract extraction in elderly patients.
Suissa S. Novel approaches to pharmacoepidemiology study design and statistical analysis. In: Strom BL, ed. Pharmacoepidemiology. 3rd ed. Chichester, United Kingdom: John Wiley & Sons,
Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic.
Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group.
Brady HR, Clarkson MR, Lieberthal W. Acute renal failure. In: Brenner BM, ed . Brenner & Rector's the kidney. 7th ed. St. Louis, MO: W B. Saunders,
Dukes MNG, Aronson JK, eds. Meyler's side effects of drugs: an encyclopedia of adverse reactions and interactions. 14th ed. Amsterdam, the Netherlands: Elsevier,
Schneeweiss S, Seeger JD, Maclure M, et al. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data.
Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data.
Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.
Clayton D, Hills M. Models for dose-response. In: Clayton D, Hills M. Statistical models in epidemiology. Oxford, United Kingdom: Oxford University Press,
Altman DG, Bland JM. Interaction revisited: the difference between two estimates.
Moride Y, Abenhaim L. Evidence of the depletion of susceptibles effect in non-experimental pharmacoepidemiologic research.
Gambaro G, Perazella MA. Adverse renal effects of anti-inflammatory agents: evaluation of selective and nonselective cyclooxygenase inhibitors.
Noroian G, Clive D. Cyclo-oxygenase-2 inhibitors and the kidney: a case for caution.
Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study.
Whelton A, White WB, Bello AE, et al. Effects of celecoxib and rofecoxib on blood pressure and edema in patients > or = 65 years of age with systemic hypertension and osteoarthritis.
Solomon DH, Schneeweiss S, Glynn RJ, et al. Relationship between selective cyclooxygenase-2 inhibitors and acute myocardial infarction in older adults.
Graham DJ, Campen D, Hui R, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study.
Kimmel SE, Berlin JA, Reilly M, et al. Patients exposed to rofecoxib and celecoxib have different odds of nonfatal myocardial infarction.
Ray WA. Evaluating medication effects outside of clinical trials: new-user designs.
Rawson NS, Nourjah P, Grosser SC, et al. Factors associated with celecoxib and rofecoxib utilization.