-
PDF
- Split View
-
Views
-
Cite
Cite
Amnon Sonnenberg, Causes underlying the birth-cohort phenomenon of peptic ulcer: analysis of mortality data 1911–2000, England and Wales, International Journal of Epidemiology, Volume 35, Issue 4, August 2006, Pages 1090–1097, https://doi.org/10.1093/ije/dyl093
Close - Share Icon Share
Abstract
Background Since humans have been infected with Helicobacter pylori for millennia, it has remained an enigma why the occurrence of gastric and duodenal ulcer rose suddenly during 19th century. The study aim is to present a mathematical model of H. pylori epidemiology that explains the peculiar long-term trends of ulcer disease.
Methods Gastric and duodenal ulcer mortality data from England and Wales between 1911 and 2000 were used to validate a model based on two simple and straightforward assumptions about H. pylori infection. First, the infection rate fell in the general population between 1800 and 2000. Second, gastric ulcer was caused by H. pylori infection contracted between the ages 5 and 15 and duodenal ulcer was caused by H. pylori infection contracted after the age of 15. As the infection receded in the general population, the two fractions of subjects who became infected between the ages 5 and 15 or after the age of 15 increased among consecutive birth cohorts.
Results The analysis of the actual long-term mortality from gastric and duodenal ulcer indicates an underlying birth-cohort pattern. These birth-cohort patterns of gastric and duodenal ulcer could be simulated by the interaction of two opposing time trends, namely a declining infection rate and a rising fraction of individuals acquiring their infection at increasingly older ages. The superimposition of a declining and a rising trend resulted in a bell-shaped curve of ulcer occurrence affecting consecutive birth-cohorts born between 1830 and 1970. Similar to the real data, the modelled cohort pattern of gastric ulcer preceded that of duodenal ulcer by 20 years.
Conclusion The birth-cohort phenomenon of ulcer disease can be explained by a receding H. pylori infection accompanied by a simultaneous shift in its age of acquisition.
The time trends of peptic ulcer disease in Western countries are characterized by an underlying birth-cohort phenomenon. The risk of development of peptic ulcer and death from it increased in consecutive generations (= birth-cohorts) born between 1840 and 1890 and decreased in all subsequent generations. The birth-cohort pattern of peptic ulcer is found to affect men and women alike.1–5 This birth-cohort phenomenon of peptic ulcer has been confirmed in numerous morbidity statistics from many different countries, including vital statistics, hospital statistics, outpatient visits, and disability pensions.6–12 A similar rise and fall among consecutive birth-cohorts is seen in gastric and duodenal ulcer, although in all statistics alike, the time trends of gastric ulcer precede those of duodenal ulcer by 10–20 years. The discovery of Helicobacter pylori (H. pylori) has shed new light on the aetiology and pathophysiology of peptic ulcer disease.13 It is now generally accepted that infection of the upper gastrointestinal tract with H. pylori is the crucial event in the development of both types of peptic ulcer. Eradication of the infection cures the majority of ulcers, unless they are caused by non-steroidal anti-inflammatory drugs or Zollinger–Ellison syndrome.14–16 Since H. pylori plays a central role in the aetio-pathogenesis of peptic ulcer, it is reasonable to assume that some underlying trends of H. pylori infection have also been responsible for the birth-cohort pattern.
Infection with H. pylori is endemic among human populations from all continents.17 Mummies of people who died thousands of years ago were shown to harbour markers of H. pylori infection.18–20 Genotyping of H. pylori strains from different populations has revealed evolutionary patterns of the bacterium that are reminiscent of ancient human migration across the globe and indicated that H. pylori must have colonized the human gastrointestinal tract since the dawn of mankind.21 The recent decline in the occurrence of peptic ulcer is well matched by a receding H. pylori infection among the general populations of all Western countries.22–24 However, the marked increase of peptic ulcer disease in the 19th century has remained an enigma. During the first half of the 19th century, physicians first came to notice the stupendous rise in the occurrence of gastric ulcer followed during the second half of the 19th century by a similarly amazing rise in the occurrence of duodenal ulcer.25,26 Overall, the sudden rise in the occurrence of both ulcer types reached epidemic proportions in many European countries. This fact has remained well documented in the medical literature, and it is still discernible in various health statistics from this time period.26–28
Several hypotheses have been put forward to explain the sudden rise of ulcer disease during the early phases of urbanization, such as the emergence of new and more virulent H. pylori strains, coinfection by multiple H. pylori strains, nutritional changes with altered susceptibility to infection, and influence of other intestinal bacteria that rendered the host more prone to develop peptic ulcer.29,30 Of all hypotheses to explain the birth-cohort phenomenon, however, the age-shift in acquisition of H. pylori provides the most convincing explanation.5,31,32 According to this hypothesis, increasing standards of hygiene shifted the age of H. pylori acquisition from early to late childhood, to adolescence and then to adulthood among consecutive generations born during the 19th century. The early generations of the 19th century, who still became infected with H. pylori as toddlers, developed chronic or atrophic pangastritis, causing a reduction of acid secretion that protected them from peptic ulcer. Gastric and duodenal ulcer could only develop in subsequent generations who contracted H. pylori during childhood and adolescence, respectively. Acquisition of H. pylori infection at older ages resulted in a more limited gastritis with less reduction of acid secretion, as in gastric ulcer, or even increased acid output of the antrum-predominant gastritis, as in most duodenal ulcers.33,34 The birth-cohort phenomenon with its initial rise during the 19th century and subsequent decline during the 20th century, therefore, would stem from the superimposition of two opposing time trends, one rising and one falling. The rise would reflect the increasing proportion of subjects who contracted H. pylori infection at an older age, while the fall would reflect the overall decline of H. pylori infection in the general population.
The present article is aimed to substantiate the hypothesis that an age-shift in H. pylori acquisition was primarily responsible for the rise of peptic ulcer during the 19th century and shows how the superimposition of two opposing trends can result in temporal patterns that closely resemble the birth-cohort phenomenon. After presenting the actual birth-cohort patterns of gastric and duodenal ulcer mortality from England and Wales, a theoretical model of H. pylori infection is developed to replicate the original trends.
Methods
Age-specific numbers of deaths from gastric ulcer and duodenal ulcer in England and Wales were obtained from the Office of Population and Census Surveys (OPCS) in London. Although the birth-cohort phenomenon of peptic ulcer has been shown to apply to various morbidity statistics of many European countries, Canada, Australia, Japan, and the United States, the vital statistics from England and Wales were chosen because they cover a relatively large population over a much longer time period than most other types of morbidity statistics. The data for the present analysis covered the time period between 1911 and 2000. The resident population of England and Wales for the same time period, broken down by age, was also from the OPCS. The two diseases were coded according to the 2nd through 10th revision of the International Classification of Diseases. For each ulcer type separately, the age-specific death rates of consecutive 10 year periods were calculated. The age-specific death rates were first plotted against the period of death to obtain the period-age contours and then against the period of birth to obtain cohort-ages contours. Age-standardized cohort mortality ratios (SCMRs) were calculated as a summary statistic of the overall mortality associated with each consecutive birth-cohort.35 This method of the indirect standardization adjusts for the changing contribution of different age groups to the mortality of different cohorts. The SCMR expressed as a percentage was plotted against the period of birth.
These four equations from above were used to model the birth-cohort pattern of gastric and duodenal ulcer.
Results
Birth-cohort pattern
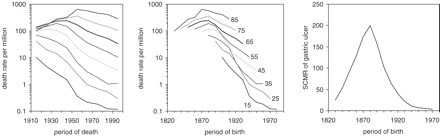
The left panel of Figure 1 displays the period-age contours of gastric ulcer in England and Wales between 1910 and 2000. Each age group and time period were labelled by their central year, for instance 55 indicating the age group 50–59 and 1975 indicating the time period 1970–79. In general, mortality from gastric ulcer tended to be higher in the older than in the younger age groups. From 1911 until 2000, the death rates from gastric ulcer declined virtually continuously in the three youngest age groups. During the same time period, the death rates increased initially in the older age groups before starting to fall. The initial rise was most pronounced in the two oldest age groups, whereas their subsequent decline was relatively smooth compared with other age groups. Overall, the period-age contours appeared to be arranged in a fan-like pattern.
Age-specific death rates of gastric ulcer in England and Wales, plotted as period-age contours (left panel), cohort-age contours (middle panel), and standardized cohort mortality ratio (SCMR, right panel). The order of age-specific curves is identical in the left and middle panel going from youngest age group at the bottom to the oldest age group at the top
The fan-like pattern of the period-age contours is difficult to explain based on changes in medical practice or standards alone. One would expect that any new and effective therapy that reduced mortality from peptic ulcer would benefit different age groups similarly. In addition, any new technique that improved diagnostic accuracy and led to more or less frequent diagnoses of peptic ulcer also should have affected the mortality in all age groups similarly. The same age-specific death rates as those depicted in the left panel of Figure 1 were plotted against the period of birth in the middle panel of Figure 1. For instance, individuals who died aged 85 between 1915 and 1995 were born between 1830 and 1910. Similarly, individuals who died aged 15 between 1915 and 1995 were born between 1900 and 1980. Plotted against the period of birth rather than the period of death, the death rates aligned as cohort-age contours cover a much longer time of 150 years as compared to the 90 years covered by the period-age contours. The cohort-age contours suggest that, irrespective of age at death, the highest mortalities from gastric ulcer occurred among those generations born around 1880.
It should be noted that the logarithmic scale of the ordinate tends to compress large changes of death rates among the older age groups and to expand small changes among the younger age groups. However, the logarithmic scale also allows one to better compare the relative changes of consecutive period-age contours and visualize them in one single graph. The underlying birth-cohort phenomenon becomes more evident, if the individual cohort specific death rates are summarized as an age-SCMR, displayed in the right panel of Figure 1. A vertical line perpendicular to the abscissa would intersect the cohort-age contours of individuals born during the same time period, but who died from gastric ulcer at a different age and during different time periods. Every point of the SCMR curve, thus, represents an approximation of the average death rate among individuals belonging to different age groups but being born during the same time period. The average mortality increased among cohorts born between 1830 and 1880. Peak mortality was reached for cohorts born in 1880 before exhibiting a marked decline.
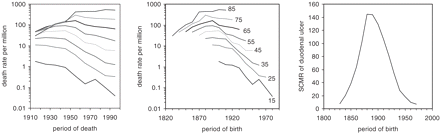
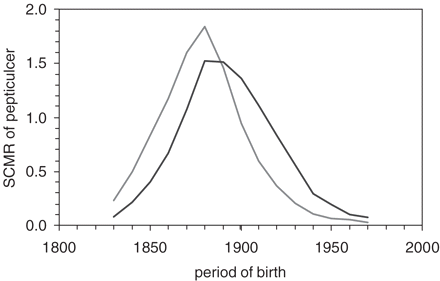
Figure 2 demonstrates a similar type of analysis for duodenal ulcer. The age-specific death rates plotted as period-age contours aligned in a fan-like appearance (left panel). The identical age-specific death rates plotted as cohort-age contours appeared to align as one hyperbola with its peak occurring in 1890 (middle panel). This optical impression of one simple pattern underlying the alignment of the individual cohort-age contours is further corroborated by calculating an average age-standardized mortality for each period of birth, as shown in the right panel. In Figure 3, the SCMR of gastric and duodenal ulcer were plotted next to each other. To facilitate comparison with the theoretical model of the next section, the SCMR was expressed as the ratio of the individual value to the overall mean of all values. Compared with gastric ulcer, the peak of duodenal ulcer appears shifted by 10–20 years towards the 20th century.
Age-specific death rates of duodenal ulcer in England and Wales, plotted as period-age contours (left panel), cohort-age contours (middle panel), and standardized cohort mortality ratio (SCMR, right panel). The order of age-specific curves is identical in the left and middle panel going from youngest age group at the bottom to the oldest age group at the top
Standardized cohort mortality ratio gastric and duodenal ulcer, calculated from the cohort-age contours shown in panels of Figures 1 and 2 and expressed as ratio of the individual value to the overall mean of all SCMR values
In the vital statistics from many other countries, the birth-cohort contours of gastric and duodenal ulcer followed a very similar pattern to the one demonstrated here for England and Wales, although the rise and subsequent decline may have been slightly shifted forward or backward on the time axis. The peaks for the birth-cohorts of gastric ulcer from different countries occurred between 1870 and 1890, whereas those for duodenal ulcer occurred between 1880 in 1900. In all countries alike, the peaks of gastric ulcer preceded those of duodenal ulcer by 10–20 years with no exception to this general pattern.40 If plotted separately, the female and male trends of each ulcer type exhibited very similar birth-cohort patterns in each individual country.1–8
Epidemiological model of the birth-cohort phenomenon
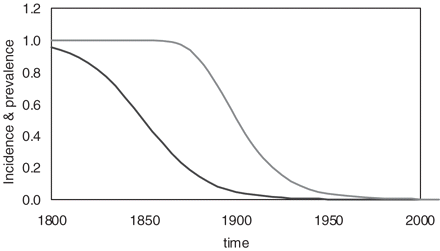
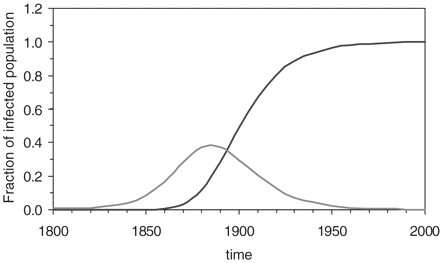
How can the birth-cohort patterns of gastric and duodenal ulcer be explained based on current knowledge of H. pylori infection? Several studies have shown that the prevalence of the infection with H. pylori is receding in the general population.22–24 In Figure 4, the time-dependent decline of the incidence rate i(t) between 1800 and 2000 was modelled by a logistic function as given by Equation (1). By inserting the time-dependent incidence rate from Equation (1) into Equation (2), one can also calculate the time-dependent changes in the prevalence of H. pylori infection among different age groups. The example of cumulative infection at age 15 years corresponds well with similar graphs published by Julie Parsonnet et al.37
Time-dependent decline in the incidence of H. pylori infection (left curve) and in the prevalence of H. pylori infection among those aged 0–15 years (right curve). The incidence was calculated using Equation (1), while the prevalence was calculated by inserting the time-dependent incidence rates from equation (1) into equation (2)
Figure 5 illustrates the fraction of patients who contracted H. pylori between the ages 5 and 15 years or after the age of 15 years. The two curves of Figure 5 were drawn according to Equations (3) and (4) using a time-dependent infection rate i(t) as given by Equation (1) and shown in Figure 4. Between 1800 and 2000, the fraction of subjects who became infected during the age interval of 5–15 years reached a peak at the turn of the century and declined subsequently. For a limited time period, when the infection was already dropping precipitously, an appreciable fraction of those subjects who harboured H. pylori had become infected during their late childhood. During the same time period, between 1800 and 2000, the fraction of subjects who became infected after the age of 15 years rose in a logistic fashion. As the infection receded in the general population, an ever increasing fraction of H. pylori infected subjects came to contract their infection at an older age (Figure 5).
Time-dependent changes in the fraction of the population infected with H. pylori between the ages 5 and 15 years (left curve) after the age of 15 years (right curve). The two fractions were calculated by inserting the incidence rates from Equation (1) into Equation (3) or Equation (4)
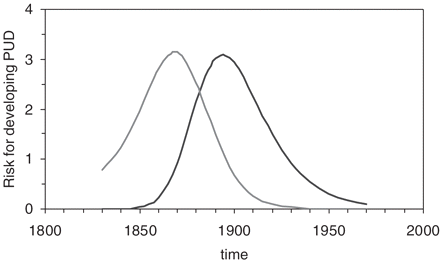
It has been proposed that gastric ulcer develops mostly in subjects who contract the infection between the age of 5 and 15 years. Similarly, duodenal ulcer appears to occur mostly in subjects who contract the infection after the age of 15 years. Accordingly, the incidence of being at risk for developing gastric ulcer would correspond with the time-dependent H. pylori incidence multiplied by the fraction of subjects infected between the ages of 5 and 15 years. The incidence of being at risk for developing duodenal ulcer would correspond to the time-dependent H. pylori incidence multiplied by the fraction of subjects infected after the age of 15 years. The resulting time trends of gastric and duodenal ulcer risk incidence are shown in Figure 6. To make the data comparable with standardized cohort mortality ratios of Figure 3, each incidence value was expressed as the ratio of the actual value over the overall mean.
Overall, a good resemblance was reached between the model and the real data as evidenced by comparing Figures 3 and 6. Similar to the real data, the cohort pattern of gastric ulcer preceded that of duodenal ulcer by 20 years. The time lag between the two curves could be shortened by changing time intervals of infection. For instance, instead of 5 and 15 years, one can use 6 and 12 years or 7 and 14 years to narrow the gap. The constants α and β of Equation (1) shift the logistic curve along the time axis and determine its slope. For the best fit with the actual data, values of α = −111 and β = 0.06 were chosen. The use of lower values for β, for instance, shifts the peaks of both ulcer types towards more recent timeperiods.
Discussion
The long-term time trends of gastric and duodenal ulcer have been shaped by a birth-cohort phenomenon with a rise in susceptibility to develop peptic ulcer among generations born during the 19th century and a subsequent decline among generations born during the 20th century. Infection of the upper gastrointestinal tract with H. pylori is the primary aetiologic mechanism precipitating the occurrence of peptic ulcer. Since H. pylori infection was endemic among all human populations for millennia until the turn of the 19th century, the cause for the sudden ulcer epidemic during the second half of the 19th century has remained an enigma. The present analysis shows how the birth-cohort pattern can be explained by the interaction of two opposing time trends, namely declining infection rate and shifting of H. pylori acquisition towards older age groups. The superimposition of a declining and a rising trend resulted in a bell-shaped peak of ulcer occurrence among consecutive birth-cohorts born between 1830 and 1970.
The present model is based on two simple and straightforward assumptions. First, the infection rate fell in the general population between 1800 and 2000. Second, peptic ulcer was caused by H. pylori infection contracted after early childhood. The first assumption is well supported by several studies that involved repetitive serologic surveillance of the general population and that documented a decrease in the prevalence of H. pylori.22–24 Similarly, the age-dependent rise in the prevalence of H. pylori in the general population represents the remnants of previously higher infection rates among the oldest generations.17,22 The second assumption rests on multiple lines of circumstantial evidence, although direct evidence for its validity is still lacking, mostly because the actual mode of infection in human populations has remained unresolved.17 First, a previous analysis of ulcer mortality in children has suggested that the risk for developing duodenal ulcer starts before the age of 15.5,41 In cases of gastric ulcer, the parallelism between the cohort-age contours of successive age groups starts at age 5–9 and, possibly, at an even younger age. In duodenal ulcer, the parallelism among cohort-age contours of successive age groups does not start before the age of 15–19 years. A regression analysis of ulcer mortality from different countries has also revealed that the environmental factors must initiate their influence before the age of 5 in gastric and before the age of 15 years in duodenal ulcer.5,41 Second, gastric and duodenal ulcer are both rare in countries where H. pylori infection rates are high and most infection is contracted during early childhood.42 Patterns of high infection rates associated with relatively low prevalence rates are observed similarly among various populations in Africa and India.43–45 Additional evidence has been provided by a case–control study among Japanese-American men in Hawaii, in whom early life acquisition of H. pylori increases the risk of development of both gastric cancer and gastric ulcer, but not duodenal ulcer.46 Third, H. pylori infection acquired during early childhood is associated with extensive gastritis, intestinal metaplasia and atrophy of the gastric mucosa, as well as gastric cancer.30 In contradistinction with peptic ulcer, the long-term time trends of gastric cancer are devoid of any rise among consecutive generations of the 19th century, but rather show a plateau or decline.47,48 Fourth, a short resurgence of an ulcer epidemic was observed in Central Europe between 1965 and 1985, when duodenal ulcers suddenly became a frequent diagnosis among migrant workers from Southern Europe.49–52 The population of migrant workers comprised mostly of young men who had left their families behind and were often housed in tight and unhygienic living quarters that may have facilitated the spread of H. pylori.53
A large body of additional evidence for the divergent influences of early vs late infection comes from pathophysiological correlations between the varying amounts of gastric acid output and the different types of infectious gastritis associated with H. pylori.33,34,54–56 In essence, peptic ulcer arises in upper gastrointestinal mucosa, which is exposed to gastric acid and whose internal defense mechanisms against the corrosive action of gastric acid have become weakened by an ongoing infection with H. pylori. Infection as a newborn or toddler most likely results in gastric atrophy with hypochlorhydria and an acid output insufficient to damage the mucosa. Infection during childhood results in chronic gastritis with a reduced capacity for acid secretion as typically found in gastric ulcer. Lastly, H. pylori infection acquired during adulthood remains often confined to the antrum and, by inhibiting the mucosal D-cells, generally results in increased acid output as typically found in duodenal ulcer.
The mathematical analysis provides a strikingly good congruence between the outcome of the model and the appearance of the actual data. Several reasons may mitigate an even more perfect match between the actual data of Figure 3 and the predicted trend of Figure 6. The model assumes a sharp cut-off between the age-acquisition of gastric and duodenal ulcer-related H. pylori infection, when in reality there might be an overlap between the two age periods. Besides the age of acquisition other factors, such as immune response to H. pylori infection and inborn variations in the amounts of gastric acid output, may also influence the predisposition to develop gastric or duodenal ulcer following an infection with H. pylori. Pyloric and prepyloric ulcers are coded as gastric ulcers but may behave epidemiologically and pathophysiologically like duodenal ulcers. (Although this presumed misclassification of peptic ulcers might blur the distinction between gastric and duodenal ulcers and their two respective peaks, the relatively small fraction of pyloric and prepyloric ulcers is unlikely to exert any large effect.) While the model makes assumptions about the incidence of gastric and duodenal ulcer, the birth-cohort pattern shown in Figure 3 relied on mortality data. The model does not account for the time trends of smoking or consumption of non-steroidal anti-inflammatory drugs and their influence on the time trends of ulcer disease. Lastly, it needs to be kept in mind that among different countries, the birth-cohort patterns of peptic ulcer exhibit some range of variation with respect to the exact temporal location of peak mortality, as well as the time lag between gastric and duodenal ulcer.1–8 The simulated pattern of Figure 6 fits well within this general pattern.
The birth-cohort phenomenon of peptic ulcer disease affected different populations at the same time. The mechanisms underlying the birth-cohort phenomenon are unlikely to have entailed a complicated interaction of multiple factors or a long chain of consecutive events, as many of these events could not have occurred in all countries alike or may have exhibited different time trends among different populations.29,30 By contradistinction, the temporal and geographic similarities of the birth-cohort phenomenon strongly argue in favour of one general epidemiologic principle that must have applied to many different countries alike. As argued in much greater detail in numerous previous publications, time trends of smoking or aspirin consumption, improvement of ulcer diagnosis through X-rays and endoscopy, more efficacious medical therapy, better surgery, and postsurgical care cannot account for the occurrence of a birth-cohort phenomenon of gastric and duodenal ulcer.5,57–60 The unifying concept of a receding H. pylori infection accompanied by a simultaneous shift in its age of acquisition secondary to increasing standards of hygiene definitely meets the criteria of ‘Ockham's razor,’ namely for the most parsimonious hypothesis to be the most likely explanation for the birth-cohort phenomenon. Since the ulcer epidemic and the associated birth-cohort phenomenon affected populations from more than a century ago, there may be no other means but epidemiologic modelling to unravel its otherwise mysterious occurrence. Besides the intellectual appeal of being able to explain historic events that have remained an enigma until now, the insights gained from the epidemiologic model also provide a lesson for present day physicians. As the relatively recent epidemic of peptic ulcer among European migrant workers showed, the persistent infection of human populations with H. pylori also entails the continued risk of peptic ulcer resurgence.
The incidence of gastric and duodenal ulcer rose throughout the 19th century, reached epidemic proportions in many European countries during the first half of the 20th century, and decreased subsequently.
Since H. pylori has been endemic in all human populations for millennia, the cause underlying this sudden rise and fall of peptic ulcer disease has remained an enigma.
As illustrated by mortality data, the rise and fall can be explained by the interaction of two opposing time trends, namely a declining infection rate and a shift in the initial acquisition of H. pylori infection towards older ages.
The superimposition of a declining (infection) trend and a rising (age) trend resulted in a bell-shaped curve of ulcer occurrence.
In retrospect, the occurrence of peptic ulcer appears like a one-time historic accident, only made possible by a receding infection of the general population with H. pylori secondary to increasing standards of hygiene in industrialized countries.
References
Sonnenberg A. Occurrence of a cohort phenomenon in peptic ulcer mortality from Switzerland.
Sonnenberg A, Müller H. Cohort and period effects in peptic ulcer mortality from Japan.
Sonnenberg A, Müller H, Pace F. Birth-cohort analysis of peptic ulcer mortality in Europe.
Sonnenberg A. Temporal trends and geographic variations of peptic ulcer disease.
Westbrook JI, Rushworth RL. The epidemiology of peptic ulcer mortality 1953–1989: a birth-chort analysis.
Svanes C, Salvesen H, Stangeland L, Svanes K, Søreide O. Perforated peptic ulcer over 56 years. Time trends in patients and disease characteristics.
Svanes C, Lie RT, Kvale G, Svanes K, Søreide O. Incidence of perforated ulcer in western Norway, 1935–1990: cohort- or period-dependent time trends?
Sonnenberg A. Disability pensions due to peptic ulcer in Germany between 1953 and 1983.
Hoogendoorn D. Opmerkelijke verschuivingen in het epidemiologische patroon van het ulcus pepticum.
Baron JH, Sonnenberg A. Period- and cohort-age contours of deaths from gastric and duodenal ulcer in New York 1804–1908.
Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration.
Marshall BJ, Goodwin CS, Warren JR et al. Prospective double-blind trial of duodenal ulcer relapse after eradication of Campylobacter pylori.
Graham DY, Lew GM, Klein PD et al. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study.
Graham DY, Lew GM, Evans DG, Evans DJ Jr, Klein PD. Effect of triple therapy (antibiotics plus bismuth) on duodenal ulcer healing. A randomized controlled trial.
Allison MJ, Bergman T, Gerszten E. Further studies on fecal parasites in antiquity.
Correa P, Fendrick AM, Allison M. The 1700-year-old mummy and its H. pylori antigens.
Suerbaum S, Achtman M. Helicobacter pylori: recombination, population structure and human migrations.
Banatvala N, Mayo K, Megraud F, Jennings R, Deeks JJ, Feldman RA. The cohort effect and Helicobacter pylori.
Cullen DJ, Collins BJ, Christiansen KJ et al. When is Helicobacter pylori infection acquired?
Parsonnet J, Blaser MJ, Perez-Perez GI, Hargrett-Bean N, Tauxe RV. Symptoms and risk factors of Helicobacter pylori infection in a cohort of epidemiologists.
Brinton W. On the Pathology, Symptoms and Treatment of Ulcer of the Stomach. London: John Churchill,
Jennings D. Perforated peptic ulcer. Changes in age-incidence and sex-distribution in the last 150 years.
Kucsko L. Das Verhalten der Ulkuskrankheit auf Grund der Sektionsprotokolle des Wiener pathologischen Institutes in den letzten 100 Jahren.
Baron JH, Sonnenberg A. Hospital admissions for peptic ulcer and indigestion in London and New York in the 19th and early 20th centuries.
Graham DY. Helicobacter pylori is the primary cause of gastric cancer.
Graham DY. Helicobacter pylori: its epidemiology and its role in duodenal ulcer disease.
Blaser MJ. Helicobacters are indigenous to the human stomach: duodenal ulceration is due to changes in gastric microecology.
Gardner MJ, Osmond C. Interpretation of time trends in disease rates in the presence of generation effects.
Taylor DN, Parsonnet J. Epidemiology and natural history of Helicobacter pylori infection. In: Blaser MJ, Smith PD, Greenberg HB, Guerrant RL, Jonathan I. Ravdin JI (eds). Infections of the Gastrointestinal Tract. Chapter 40. New York: Raven Press,
Parsonet J. The incidence of Helicobacter pylori infection.
Cucino C, Sonnenberg A. The long-term time trends of ulcerative colitis and peptic ulcer are interrelated.
Sonnenberg A. Causative factors in the etiology of peptic ulcer disease become effective before the age of 15 years.
Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries.
Tovey FI, Tunstall M. Duodenal ulcer in black populations in Africa south of the Sahara.
Blaser MJ, Chyou PH, Nomura A. Age at establishment of Helicobacter pylori infection and gastric carcinoma, gastric ulcer, and duodenal ulcer risk.
Sonnenberg A. The US temporal and geographic variations of diseases related to Helicobacter pylori.
Office of Population Censuses and Surveys. Cancer Statistics—Incidence, Survival and Mortality in England and Wales. Studies on Medical and Population Subjects No. 43. London: Her Majesty's Stationery Office,
Quaquish I, Burkhardt HU, Heilmann KL. Magenerkrankungen bei ausländischen Arbeitnehmern in der Bundesrepublik Deutschland.
Würsch TG, Hess H, Walser R et al. Die Epidemiologie des Ulcus duodeni. Untersuchungen an 1105 Patienten in Zürich.
Schmid E, Vollmer K, Allmendinger G, Blaich E, Hofgärtner F. Epidmiologische Resultate der endoskopischen Untersuchungen beim Ulcus ventriculi und Ulcus duodeni.
Gutierrez O, Melo M, Segura AM, Angel A, Genta RM, Graham DY. Cure of Helicobacter pylori infection improves gastric acid secretion in patients with corpus gastritis.
El-Omar EM, Oien K, El-Nujumi A et al. Helicobacter pylori infection and chronic gastric acid hyposecretion.
Katelaris PH, Seow F, Lin BP, Napoli J, Ngu MC, Jones DB. Effect of age, Helicobacter pylori infection, and gastritis with atrophy on serum gastrin and gastric acid secretion in healthy men.
Sonnenberg A. Geographic and temporal variations in the occurrence of peptic ulcer disease.
Sonnenberg A, Cucino C, Bauerfeind P. The unresolved mystery of birth-cohort phenomena in Gastroenterology.
Sonnenberg A. A personal history of giving birth to the cohort phenomenon. In: Marshall BJ (ed.). Helicobacter Pioneers: First Hand Accounts from the Scientists Who Discovered Helicobacters, 1892–1982. Blackwell Science, Oxford, UK,