-
PDF
- Split View
-
Views
-
Cite
Cite
Przemyslaw Kardas, Comparison of patient compliance with once-daily and twice-daily antibiotic regimens in respiratory tract infections: results of a randomized trial, Journal of Antimicrobial Chemotherapy, Volume 59, Issue 3, March 2007, Pages 531–536, https://doi.org/10.1093/jac/dkl528
Close - Share Icon Share
Abstract
Patient compliance seems to be highly dependent on the number of daily doses. However, it is unclear whether this effect is still present in the case of infrequent regimens during short-term antibiotic treatment. The aim of this study was to find out whether a once-daily antibiotic regimen provides better patient compliance in the case of common respiratory tract infections than a twice-daily regimen.
Outpatients with acute bacterial exacerbation of chronic bronchitis were treated with clarithromycin 250 mg twice daily or clarithromycin in modified release formulation 500 mg once daily, for 7 days in a prospective, randomized, single-centre study. Patient compliance was assessed with electronic monitoring.
Of 122 patients randomized, 119 were evaluable (58 in the once-daily group and 61 in the twice-daily group). All the studied parameters indicated significantly better compliance with the once-daily versus twice-daily antibiotic formulation: overall compliance (93.7% versus 81.3%, P < 0.0001), days with correct number of doses taken (80.3% versus 68.6%, P < 0.0001), correct interdose intervals (74.4% versus 56.4%, P < 0.001), and the mean interdose intervals (95.6% versus 106.3% of the expected values, P < 0.001).
The study has proved much better patient compliance with a once-daily versus a twice-daily antibiotic regimen. This effect has been marked in both dosing and timing compliance. These findings indicate the clinical usefulness of a once-daily antibiotic regimen in assuring patient compliance during the treatment of respiratory tract infections.
Introduction
Patient compliance is crucial for successful therapy. However, even when the condition is symptomatic and patients are well aware of the consequences, some do not adhere to the prescribed treatment. It is estimated that in general at least 50% of patients fail to receive full treatment benefit due to inadequate compliance.1 This, in turn, leads to profound consequences including the need for additional procedures or treatment, and contributes to hospital admissions.2,3
This behaviour is also a factor markedly limiting the effectiveness of antibiotic treatment in ambulatory care settings.4 According to a recent meta-analysis, as many as 37.8% of patients fail to take some of the prescribed antibiotic doses, despite the fact that the therapy is usually short and intensified symptoms should motivate patients to follow doctors' instructions.5 The consequences of patient non-compliance in the case of antibiotic therapy may not only lead to treatment failure but also to relapses and complications. From both individual and population perspectives, inadequate compliance also favours the emergence of bacterial resistance.6 All these consequences contribute to its enormous cost burden, thus making non-compliance with antibiotics not only a serious medical problem, but also a social one.
Contrary to common expectations, factors such as intelligence, memory, age, education or the number of drugs a patient takes seem not to affect the level of adherence.7 On the other hand, the effect of the number of daily doses on compliance has been found previously in various clinical conditions. It seems to be a rule that the fewer the daily doses, the better the compliance.8 Nevertheless, there is still lack of direct proof of effectiveness of once-daily over twice-daily regimens in providing better patient compliance with antibiotics. This dilemma is of high practical importance, as most antibiotics are currently available in once-daily or twice-daily formulations. Therefore, the aim of this trial was to compare patient compliance with a once-daily versus a twice-daily antibiotic regimen in the case of community-acquired acute respiratory tract infection (RTI). To avoid problems of inadequacy, unreliability and no insight into detailed timing of doses, which are connected with subjective assessment methods, the study was based on electronic compliance assessment, giving a unique chance to study detailed dosing and timing history of antibiotic administration.6,9,10
Patients and methods
Patients and study design
This was a prospective, randomized, single-centre study, investigating compliance in patients treated with once-daily or twice-daily antibiotic regimens owing to acute bacterial RTI. The study protocol was approved by the Ethics Committee of the Medical University of Lodz.
Outpatients diagnosed with acute bacterial exacerbation of chronic bronchitis (ABECB) were enrolled into the study. The ABECB diagnosis was established by primary-care physicians treating the individuals suffering from chronic bronchitis, based on modified Anthonisen criteria, i.e. at least two of six of the following parameters: increased cough, increase in sputum volume, altered sputum clarity, presence of wheezing, chills and/or body temperature ≥ 37.5°C, and emergence/worsening of dyspnoea.11 The inclusion criteria also included age 18 to 60 years, ability to work prior to the infection, mental state enabling conscious participation in the study and conscious consent form signed.
The main exclusion criteria were antibiotic treatment during the same episode of exacerbation before entering the study, unstable angina pectoris, New York Heart Association class III and IV heart failure, unstable diabetes, advanced renal or liver failure, any conditions requiring the help of others with drug administration (e.g. manual disability, impaired eyesight, etc.), and any known contraindication to clarithromycin treatment.
The sample size of 120 patients in the trial was obtained by assuming overall compliance (primary outcome measure) difference between the groups of 20%, with a power of 80% and α = 0.05.12
Patients were recruited to the trial by their primary-care physicians, and were informed about the aim and methods of the study. Then, they were randomly assigned to clarithromycin (Klacid, Abbott S. p. A., Italy) 250 mg twice daily or clarithromycin in modified release formulation (Klacid UNO, Abbott Laboratories, UK) 500 mg once daily, for 7 consecutive days. An independent statistical centre (Department of Medical Statistics, Medical University of Lodz) prepared a random table for the study; after recruiting a patient the centre was contacted and the allocation was given to the investigator. Study drugs were given to the patients in excessive amount, in MEMS 6 containers (Medication Event Monitoring System, Aardex Ltd, Zug, Switzerland), which consist of a standard tablet bottle and a cap containing a microprocessor that registers the date and time of every opening and enables precise and objective electronic measurement of compliance parameters.13 The patients were instructed on how to use a MEMS container correctly, i.e. to open it for no other reason but to take out tablets directly before use. Patients were also asked to return containers to their doctors with all the remaining tablets inside during the control visit at day 7.
Data processing and statistical analysis
After completion of the treatment period, MEMS containers were collected from the patients. Data from the containers were transferred into a computer and processed using the PowerView v. 1.3.2 program (Aardex Ltd).
For the purpose of further calculations, multiple MEMS openings within a short period ( ≤ 15 min) were filtered and not counted. All other recorded openings were considered to represent a single dose intake. Categorical variables were compared using a χ2 test. Nominal data were expressed as means, medians and interquartile ranges and were compared using the Mann–Whitney test. The statistical significance threshold was chosen at P < 0.05.
Compliance measurement
The following MEMS-derived parameters were employed for patient compliance assessment: The clinical outcomes were ascertained, as well, but will be reported in detail elsewhere.
Overall compliance (primary outcome measure), defined as the ratio of the number of container openings to the number of prescribed doses.
Days with correct number of doses taken (i.e. days with one and two container openings daily in the case of once-daily and twice-daily regimens, respectively).
Correct interdose intervals, defined as the intervals between consecutive container openings in a range of 11–13 h after the previous MEMS 6 opening in the case of the twice-daily regimen and 22–26 h in the case of the once-daily regimen.
Mean interdose intervals, expressed as the ratio of mean interdose intervals to the relevant expected interdose interval.
Results
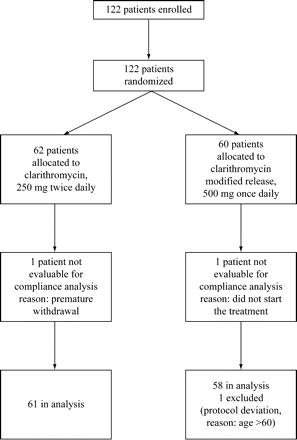
The number of eligible patients was not recorded. Altogether, 122 patients were enrolled and randomized. Of these, 60 (49.2%) belonged to the once-daily group and 62 (50.8%) to the twice-daily group (Figure 1). The patients' demographic characteristics are given in Table 1. The two groups did not differ in terms of both age and sex. The compliance analysis was performed for the per protocol group, i.e. the 119 patients who completed the full study course and for whom all data was available.
Demographic characteristics of studied groups on presentation
| Variable . | Together . | Once-daily group . | Twice-daily group . |
|---|---|---|---|
| No. of patients | 122 | 60 | 62 |
| Gender, n (%) | |||
| Male | 52 (42.6) | 29 (48.3) | 23 (37.1) |
| Female | 70 (57.3) | 31 (51.7) | 39 (62.9) |
| Age (years) | |||
| Mean | 46.6 | 47.8 | 45.5 |
| Standard deviation | 10.6 | 10.6 | 10.5 |
| Signs and symptoms, n (%) | |||
| Increase in frequency of cougha | 118 (97) | 57 (95) | 61 (98) |
| Increase in severity of cougha | 117 (96) | 57 (95) | 60 (97) |
| Increase in daily volume of sputum productiona | 106 (87) | 51 (85) | 55 (89) |
| Increase in sputum purulencea | 91 (75) | 47 (78) | 44 (71) |
| New onset of, or increasea in dyspnoea | 58 (48) | 26 (43) | 32 (52) |
| New onset of, or increasea in chest congestion, indicated by the presence of adventitious sounds (rales, rhonchi and/or wheezes) | 87 (71) | 42 (70) | 45 (73) |
| Increasea in breath sound intensity | 92 (75) | 44 (73) | 48 (77) |
| New onset of, or increasea in prolongation of the expiratory phase | 62 (51) | 33 (55) | 29 (47) |
| Body temperature ≥ 37.5°C | 79 (65) | 40 (67) | 39 (63) |
| Variable . | Together . | Once-daily group . | Twice-daily group . |
|---|---|---|---|
| No. of patients | 122 | 60 | 62 |
| Gender, n (%) | |||
| Male | 52 (42.6) | 29 (48.3) | 23 (37.1) |
| Female | 70 (57.3) | 31 (51.7) | 39 (62.9) |
| Age (years) | |||
| Mean | 46.6 | 47.8 | 45.5 |
| Standard deviation | 10.6 | 10.6 | 10.5 |
| Signs and symptoms, n (%) | |||
| Increase in frequency of cougha | 118 (97) | 57 (95) | 61 (98) |
| Increase in severity of cougha | 117 (96) | 57 (95) | 60 (97) |
| Increase in daily volume of sputum productiona | 106 (87) | 51 (85) | 55 (89) |
| Increase in sputum purulencea | 91 (75) | 47 (78) | 44 (71) |
| New onset of, or increasea in dyspnoea | 58 (48) | 26 (43) | 32 (52) |
| New onset of, or increasea in chest congestion, indicated by the presence of adventitious sounds (rales, rhonchi and/or wheezes) | 87 (71) | 42 (70) | 45 (73) |
| Increasea in breath sound intensity | 92 (75) | 44 (73) | 48 (77) |
| New onset of, or increasea in prolongation of the expiratory phase | 62 (51) | 33 (55) | 29 (47) |
| Body temperature ≥ 37.5°C | 79 (65) | 40 (67) | 39 (63) |
NS: P > 0.05
aCompared with the state before chronic bronchitis exacerbation.
Demographic characteristics of studied groups on presentation
| Variable . | Together . | Once-daily group . | Twice-daily group . |
|---|---|---|---|
| No. of patients | 122 | 60 | 62 |
| Gender, n (%) | |||
| Male | 52 (42.6) | 29 (48.3) | 23 (37.1) |
| Female | 70 (57.3) | 31 (51.7) | 39 (62.9) |
| Age (years) | |||
| Mean | 46.6 | 47.8 | 45.5 |
| Standard deviation | 10.6 | 10.6 | 10.5 |
| Signs and symptoms, n (%) | |||
| Increase in frequency of cougha | 118 (97) | 57 (95) | 61 (98) |
| Increase in severity of cougha | 117 (96) | 57 (95) | 60 (97) |
| Increase in daily volume of sputum productiona | 106 (87) | 51 (85) | 55 (89) |
| Increase in sputum purulencea | 91 (75) | 47 (78) | 44 (71) |
| New onset of, or increasea in dyspnoea | 58 (48) | 26 (43) | 32 (52) |
| New onset of, or increasea in chest congestion, indicated by the presence of adventitious sounds (rales, rhonchi and/or wheezes) | 87 (71) | 42 (70) | 45 (73) |
| Increasea in breath sound intensity | 92 (75) | 44 (73) | 48 (77) |
| New onset of, or increasea in prolongation of the expiratory phase | 62 (51) | 33 (55) | 29 (47) |
| Body temperature ≥ 37.5°C | 79 (65) | 40 (67) | 39 (63) |
| Variable . | Together . | Once-daily group . | Twice-daily group . |
|---|---|---|---|
| No. of patients | 122 | 60 | 62 |
| Gender, n (%) | |||
| Male | 52 (42.6) | 29 (48.3) | 23 (37.1) |
| Female | 70 (57.3) | 31 (51.7) | 39 (62.9) |
| Age (years) | |||
| Mean | 46.6 | 47.8 | 45.5 |
| Standard deviation | 10.6 | 10.6 | 10.5 |
| Signs and symptoms, n (%) | |||
| Increase in frequency of cougha | 118 (97) | 57 (95) | 61 (98) |
| Increase in severity of cougha | 117 (96) | 57 (95) | 60 (97) |
| Increase in daily volume of sputum productiona | 106 (87) | 51 (85) | 55 (89) |
| Increase in sputum purulencea | 91 (75) | 47 (78) | 44 (71) |
| New onset of, or increasea in dyspnoea | 58 (48) | 26 (43) | 32 (52) |
| New onset of, or increasea in chest congestion, indicated by the presence of adventitious sounds (rales, rhonchi and/or wheezes) | 87 (71) | 42 (70) | 45 (73) |
| Increasea in breath sound intensity | 92 (75) | 44 (73) | 48 (77) |
| New onset of, or increasea in prolongation of the expiratory phase | 62 (51) | 33 (55) | 29 (47) |
| Body temperature ≥ 37.5°C | 79 (65) | 40 (67) | 39 (63) |
NS: P > 0.05
aCompared with the state before chronic bronchitis exacerbation.
No multiple MEMS openings within a period of ≤ 15 min were observed and therefore all the openings were considered to represent a single dose intake and used for calculations.
All the studied parameters indicated significantly better compliance with the once-daily versus twice-daily antibiotic formulation (Table 2): overall compliance (93.7% versus 81.3%, P < 0.0001), days with correct number of doses taken (80.3% versus 68.6%, P < 0.0001), correct interdose intervals (74.4% versus 56.4%, P < 0.001), as well as the mean interdose intervals (95.6% versus 106.3% of the expected values, P < 0.001). There were 27 no-dose days in the once-daily group (6.7%) versus 19 in the twice-daily group (4.5%); the difference is not statistically significant (P > 0.05).
Parameters of patient compliance in studied groups
| Regimen . | Overall compliance (%) . | Days with correct number of doses (%) . | Correct interdose intervals (%) . | Mean interdose interval (% of norm) . |
|---|---|---|---|---|
| Once daily (n = 58) | ||||
| Mean | 93.7** | 80.3** | 74.4* | 95.6* |
| Median | 87.5 | 87.5 | 85.7 | 99.5 |
| Interquartile range Q1–Q3 | 87.5–100.0 | 75.0–87.5 | 57.1–85.7 | 91.9–100.0 |
| Twice daily (n = 61) | ||||
| Mean | 81.3** | 68.6** | 56.4* | 106.3* |
| Median | 81.3 | 75.0 | 64.3 | 100.1 |
| Interquartile range Q1–Q3 | 78.6–87.5 | 62.5–85.7 | 35.7–78.6 | 99.4–108.5 |
| Regimen . | Overall compliance (%) . | Days with correct number of doses (%) . | Correct interdose intervals (%) . | Mean interdose interval (% of norm) . |
|---|---|---|---|---|
| Once daily (n = 58) | ||||
| Mean | 93.7** | 80.3** | 74.4* | 95.6* |
| Median | 87.5 | 87.5 | 85.7 | 99.5 |
| Interquartile range Q1–Q3 | 87.5–100.0 | 75.0–87.5 | 57.1–85.7 | 91.9–100.0 |
| Twice daily (n = 61) | ||||
| Mean | 81.3** | 68.6** | 56.4* | 106.3* |
| Median | 81.3 | 75.0 | 64.3 | 100.1 |
| Interquartile range Q1–Q3 | 78.6–87.5 | 62.5–85.7 | 35.7–78.6 | 99.4–108.5 |
Due to the study design, there are double the number of observations in the twice-daily arm.
*P < 0.001; **P < 0.0001.
Parameters of patient compliance in studied groups
| Regimen . | Overall compliance (%) . | Days with correct number of doses (%) . | Correct interdose intervals (%) . | Mean interdose interval (% of norm) . |
|---|---|---|---|---|
| Once daily (n = 58) | ||||
| Mean | 93.7** | 80.3** | 74.4* | 95.6* |
| Median | 87.5 | 87.5 | 85.7 | 99.5 |
| Interquartile range Q1–Q3 | 87.5–100.0 | 75.0–87.5 | 57.1–85.7 | 91.9–100.0 |
| Twice daily (n = 61) | ||||
| Mean | 81.3** | 68.6** | 56.4* | 106.3* |
| Median | 81.3 | 75.0 | 64.3 | 100.1 |
| Interquartile range Q1–Q3 | 78.6–87.5 | 62.5–85.7 | 35.7–78.6 | 99.4–108.5 |
| Regimen . | Overall compliance (%) . | Days with correct number of doses (%) . | Correct interdose intervals (%) . | Mean interdose interval (% of norm) . |
|---|---|---|---|---|
| Once daily (n = 58) | ||||
| Mean | 93.7** | 80.3** | 74.4* | 95.6* |
| Median | 87.5 | 87.5 | 85.7 | 99.5 |
| Interquartile range Q1–Q3 | 87.5–100.0 | 75.0–87.5 | 57.1–85.7 | 91.9–100.0 |
| Twice daily (n = 61) | ||||
| Mean | 81.3** | 68.6** | 56.4* | 106.3* |
| Median | 81.3 | 75.0 | 64.3 | 100.1 |
| Interquartile range Q1–Q3 | 78.6–87.5 | 62.5–85.7 | 35.7–78.6 | 99.4–108.5 |
Due to the study design, there are double the number of observations in the twice-daily arm.
*P < 0.001; **P < 0.0001.
The time history of patient compliance showed a similar pattern of compliance changes with time in both study arms: compliance reached its maximum on day 3 and then subsequently dropped, still being markedly better for the once-daily group (Figure 2).
Patient compliance over time: percentage of patients who opened the MEMS container the satisfactory number of times a day [i.e. at least once for the once daily (OD) regimen, and at least twice for the twice daily (BD) regimen].
The rate of clinical cure or improvement (defined as at least 50% of initial signs resolved or reduced at day 7) was high and not significantly different between the groups (94.5% in the once-daily group versus 88.1% in the twice-daily group, P > 0.05).
Discussion
In this study, significantly better compliance with antibiotic dosing and dose timing was observed with a once-daily regimen compared with a twice-daily regimen. Due to the objective method of electronic monitoring, the strength of this study is that, for the first time, it has been possible to compare precisely patient compliance between regimens to prove a possible advantage for once-daily dosing. To date, only a few studies have explored the difference in compliance between once and twice daily antibiotic regimens,14 with once-daily regimens providing better levels of compliance. However, these results are not fully convincing because, as well as pill counts, subjective assessment techniques were used (patient reports etc.), which have frequently been proven to seriously underestimate non-compliance.10,15 Similarly, only a limited number of studies so far have used electronic monitoring to assess antibiotic compliance, of which only two dealt with the treatment of RTIs;16,17 the others dealt with sexually transmitted diseases,18–20Helicobacter pylori eradication21,22 and sickle cell disease.23
This study has some limitations. Due to ethical reasons, all patients were informed about the aim of the trial. This knowledge about the monitoring may have slightly increased compliance when compared with daily practice, although this assumption has not been confirmed in other studies.24 Patients also received more tablets than were needed. However, the difference in compliance between the groups cannot be attributed to these factors as both study arms were conducted under the same conditions. Finally, due to the study design, there were twice as many observations in the twice-daily arm.
Several factors contribute to patient non-compliance with antibiotic therapy: out-of-pocket cost of the drug, the formulation, a rapid improvement of symptoms, forgetfulness, side effects and patients' beliefs.4,25,26 However, it seems to be a rule that the fewer the daily doses the better the compliance.8
On the other hand, good dosing compliance is not always followed by satisfying dose timing, as observed by Favre et al.16 in the only study to assess this aspect of antibiotic compliance. In that study, which used a twice-daily regimen, only 32.6% of doses were taken within 12 ± 1 h of the previous dose. Interestingly, the present study is the first to prove much better compliance with dose timing with once-daily versus twice-daily antibiotic regimens.
In some comparative studies of once- and twice-daily dosing, better compliance for a once-daily regimen was accompanied by a higher percentage of no-dosing days. In a cross-over study comparing calcium channel blockers administered once and twice daily, taking compliance improved in 30% of patients when switching from a twice-daily to a once-daily regimen, but, at the same time, there was a 15% increase in the number of patients with one or more no-dosing days.27 For this reason, the twice-daily regimen was considered superior to the once-daily regimen by some authors.28,29 Contrary to these findings, in the present study, there was only a slight, statistically insignificant difference with respect to no-dosing days between the once-daily and twice-daily groups.
The current study also demonstrated that patient compliance reaches a maximum within the initial 3 days of antibiotic treatment and then reduces over time, confirming similar previous observations.30,31 This reflects well-known patient behaviour with chronic treatment, but in the acute therapy studied was unexpectedly rapid. The natural consequence of that finding should be shortening of the treatment period to the minimum. Recently, a number of trials have found shorter antibiotic regimens clinically effective and not inferior to more traditional, longer therapies.32–34
One possible explanation of why once-daily dosing is superior to a twice-daily regimen is the ease with which the morning dose can be tailored to routine patient activities. Indeed, it has been frequently observed with twice-daily regimens that the morning doses are taken more precisely than the evening ones.35,36
When both the number of daily doses and the length of treatment are taken into consideration, the impact of different therapies on compliance may be especially striking. In a study comparing short, 3 day therapy with once-daily azithromycin with 10 day standard treatment with three times daily penicillin V in the treatment of acute group A streptococcal tonsillopharyngitis, rates of compliance of 94–95% were observed with azithromycin, compared with only 62% with penicillin V.37 Once-daily, short-course treatment also better satisfies patients' expectations, as it is perceived to be more effective than longer antibiotic courses.26,38 Indeed, compliance improves remarkably with shorter and less-frequent regimens, as observed in the current study. Therefore, the novel single oral high-dose azithromycin therapy, recently introduced to treat RTIs, seems to be very promising in terms of overcoming problems with compliance by using the simplest possible outpatient regimen.39
Of course, once-daily antibiotic dosing is not a panacea and is not always associated with better outcomes: one meta-analysis demonstrated that once-daily penicillin is associated with decreased efficacy compared with more frequent dosing in the treatment of streptococcal tonsillopharyngitis.40 The current study also failed to demonstrate a statistically significant difference in clinical outcomes between once- and twice-daily regimens. Therefore, larger trials are required to answer this question unequivocally. Finally, the reasons for non-compliant patient behaviour are numerous, and therefore other techniques may be helpful to address the problem, such as reminders, telephone follow-up etc.41–43
There is still an open question over how doctors should behave when mindful of the problem of patient non-compliance with antibiotic therapy. Recently, the whole concept of compliance itself has been criticized for its paternalism. Instead, a new approach, namely concordance, was advocated ‘to describe the state of agreement achieved by therapeutic alliance reached through negotiations, in which both parties, i.e. doctor and patient, are equal’.44,45 However, it is ultimately the doctor who takes the legal, ethical and professional responsibility for following guidelines and providing the patient with proven and effective treatment. Therefore, at least in the case of acute antibiotic treatment, there is limited scope for negotiation with the patient as to the acceptable frequency and duration of the regimen. Instead, it seems reasonable to use strategies aimed at enhancing patient compliance. For that reason, and in the light of the data presented here, a once-daily regimen can be considered an attractive option for both doctors and patients when prescribing antibiotic therapy.
Acknowledgements
The participation of the following primary care physicians is gratefully acknowledged: Maciej Baranski, Hanna Boguszewska, Tomasz Gula, Rafal Mazur, Magdalena Muras, Jacek Plucinski, Agata Plusa-Zak and Hubert Stefan. Editorial support was kindly provided by Keith Littlewood. Funding for this study was provided by a scientific grant from the Medical University of Lodz (no. 502-16-251). No additional support was provided in any form for this study; this includes no support from the pharmaceutical industry.
Transparency declarations
None to declare.



![Patient compliance over time: percentage of patients who opened the MEMS container the satisfactory number of times a day [i.e. at least once for the once daily (OD) regimen, and at least twice for the twice daily (BD) regimen].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jac/59/3/10.1093/jac/dkl528/2/m_dkl52802.gif?Expires=1716323910&Signature=WrUXmWdMm1-a8YBX6H4-Brwkij-ScMR669jYLQ66FvC4cugUnuCvyCFvajhTDQj00gdoc8Yvi7cvajPYtzcHrcObl-fxuY58BG-wJNMfQRZ7s~1hadMMnomSbo6tSdf1ePQ~VQz-ncw5OQCKRaJMMUOa92akcHsNyoceEYr-d7BMJ2vXQehSoH3N8XQxx2v9ebenhh3NdeblXQZpU2UuOnQkhAWsY4VzLibYKRNbQpQqwJKfEplQuawBE7ILaRwShg4OHMMSbXt59L8bEAhMNmPddifMgdCIkiyIqpZ~PfvMn4oIM834igWxoAwod5EKD7ZskR~6YH1rMAZE6-b27Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
