-
PDF
- Split View
-
Views
-
Cite
Cite
Chris Jones, Paul Roderick, Scott Harris, Mary Rogerson, Decline in kidney function before and after nephrology referral and the effect on survival in moderate to advanced chronic kidney disease, Nephrology Dialysis Transplantation, Volume 21, Issue 8, August 2006, Pages 2133–2143, https://doi.org/10.1093/ndt/gfl198
Close - Share Icon Share
Abstract
Background. The burden of chronic kidney disease (CKD) is high, but its natural history and the benefit of routine nephrology care is unclear. This study investigated the decline in kidney function prior to and following nephrology referral and its association with mortality.
Methods. This study provides a retrospective review of the individual rates of glomerular filtration rate (GFR) decline (millilitre per minute per 1.73 m2/year) for the 5 years before and after referral in 726 new referrals with stages 3–5 CKD to one renal unit between 1997 and 2003. Blood pressures are averages at referral, 1 and 3 years post referral. Logistic regression and Cox's models tested factors predicting post-referral GFR decline and the impact on mortality.
Results. Mean (SD) age was 72 (14), and 389 (54%) patients had stages 4–5 CKD. GFR decline slowed significantly from −5.4 ml/min/1.73 m2/year (−13. to −2) before to −0.35 ml/min/1.73 m2/year (−3 to +3) after referral (P < 0.001). Blood pressure also reduced significantly (155/84 to 149/80, P < 0.05) with most changes occurring within 1 year of referral. Factors predicting a non-progressive post-referral decline included a lower systolic blood pressure at referrral and 1 year after referral, a CKD diagnosis other than diabetic nephropathy, less baseline proteinuria and a non-progressive pre-referral GFR decline. A non-progressive post-referral GFR decline was independently associated with significantly better survival (hazard ratio 0.55, 95% CI 0.40–0.75, P ≤ 0.001) after adjustment for known risk factors.
Conclusions. Following nephrology referral, GFR decline slowed significantly and was associated with better survival. Earlier detection of patients with progressive CKD and interventions to slow progression may have benefits on both kidney and patient survival.
Introduction
Chronic kidney disease (CKD) is now recognized to be a relatively common condition [1,2] associated with considerable morbidity and mortality especially in its later stages. If established renal failure (ERF) develops and renal replacement therapy (RRT) is commenced, the costs are substantial [3] and are forecast to rise [4] in line with predictions of a continued increase in the prevalence of RRT patients [5–10]. This has fuelled interest in measures to delay the progression to ERF [11,12]. However, the natural history of CKD is not well understood. CKD is not inevitably progressive [13], and those with kidney disease may be more likely to die than reach ERF largely due to the high cardiovascular risk associated with CKD [14,15]. Whilst interventional studies have shown the effectiveness of blood pressure (BP) control [16], and specific therapies such as angiotensin-converting enzyme inhibitors (ACEIs) in slowing CKD progression and reducing mortality [17,18], these are in selected groups of study patients with strict entry criteria, BP targets and frequent assessments that may not reflect usual care. Consequently, the evidence of the benefit of routine nephrology care in slowing CKD progression is limited [19,20] and mostly assessed retrospectively in those who start RRT.
This article presents data from a retrospective observational study of patients with moderate to advanced stage 3–5 CKD, investigating how effective routine nephrology care was in slowing the rate of progression of CKD and whether this was associated with patient survival.
Subjects and methods
Patients and data collection
Between 1997 and 2003, 947 patients were referred to the renal unit in Southampton, UK, with stages 3–5 CKD. Baseline and follow-up characteristics and outcomes were collected for all, though only 726 patients had sufficient serum creatinine tests before and after the referral to calculate a rate of decline in kidney function. The analysis was restricted to these 726 patients.
This unit accepts patients from primary and secondary care with no local criteria in place to guide referral, although there is a Shared Primary and Nephrology Care Scheme which monitors patients in partnership with Primary Care [21], and this may make general practitioners more aware of nephrological management issues. This renal unit's database has access to all haematological and biochemical tests taken for each patient before and after referral. In our unit, laboratory testing of urinary protein excretion is only carried out if urinary dipstick analysis shows more than or equal to ‘1+’. As such, no baseline laboratory values were available in those with a dipstick less than ‘1+’. For the purpose of this study, these patients were classified as having a urinary protein excretion of less than 300 mg/day [22], having 300 mg to 1 g/day and >1 g/day according to laboratory values. A detailed summary of patients’ clinical state, prior medications and interventions at first referral and during follow-up were obtained from the computerized referral and follow-up correspondence. Deaths before RRT and progression to RRT were routinely recorded on the database but the cause of death was not available. Median inter-quartile range (IQR) for follow-up was 2.9 (1.3–4.1) years.
Study objectives
To describe the decline in GFR up to 5 years prior to and following first referral to nephrologists and to identify the factors associated with a slow GFR decline post referral.
To describe the outcomes in terms of RRT and death before RRT and the impact of a slow GFR decline on survival after adjustment for other predictive factors.
Definitions
The UK Renal Association standards for BP (<140/80 mmHg), serum haemoglobin (>10 g/dl), albumin (>30 g/dl), calcium (2.2–2.6 mmol/l) and phosphate (<1.9 mmol/l) were used [23]. The GFR was calculated using the Modification of Diet in Renal Disease (MDRD) study four-item equation [186.3 × (serum creatinine)−1.154 × (age)−0.203 × 1.212 (if patient is black) × 0.742 (if female)] [24,25]. Virtually, all testing of serum creatinine was carried out in one laboratory using a modified Jaffe reaction. Correction to the MDRD laboratory method was not carried out. Serum albumin was tested using the Bayer Advia 1650 and bromocresol purple with normal limits of 32–47 g/l. The stages of CKD from mild (stage 1) to advanced (stage 5) are those devised by the Kidney Disease Outcomes Quality Initiative (KDOQI) group [26]. Patients were classified as having a vascular disease at baseline if they had documented evidence of either cardiovascular (myocardial infarction, angina, coronary revascularization), cerebro-vascular (stroke, transient ischaemic attack) or peripheral vascular disease. Data on certain cardiovascular risk factors were incomplete including smoking, lipid levels and obesity. Diabetic nephropathy was considered the cause of CKD if proteinuria was >1 g/day and/or retinopathy was present with no other obvious cause apparent. Hypertensive nephropathy was a clinical diagnosis made in those with small kidneys, no overt proteinuria and no other obvious cause.
Change in BP
Systolic (SBPs) and diastolic (DBPs) blood pressures at 1 and 3 years after nephrology referral were the average of the measures recorded within 1 month of each time point. Mean BPs at these time points were calculated to assess when any changes occurred and the impact on decline in GFR.
Decline in MDRD GFR
At least three GFR measures during the 5 years before and the 5 years after referral were required to estimate a pre- and post-referral GFR slope. A total of 726 patients fulfilled these criteria and the regression coefficient of time against GFR was used to give an estimated rate of GFR decline in millilitre per minute per 1.73 m2/year for each individual patient. These were then averaged to achieve a summary rate. Overall, the pre- and post-referral slopes were based on a median (IQR) of 11 (5–23) and 15 (8–28) individual GFR measures, respectively.
The KDOQI clinical practice guidelines suggest that GFR is estimated to reduce by ∼1 ml/min/1.73 m2/year from a normal level of 125 ml/min/1.73 m2 once adulthood is reached [12,27]. Therefore, any reduction greater than this was considered to reflect progression of CKD [slope more negative than −1 ml/min/1.73 m2/year (≤−1)] or non-progressive if the slope is less negative than −1.0 ml/min/1.73 m2/year(>−1).
The slopes were further subdivided into ‘fast progressive’ [more negative than −5 (≤−5)], ‘slow progressive’ (>−5 to ≤−1), ‘stable’ (>−1 to ≤+1), ‘moderately non-progressive’ (>+1 to <+5) and ‘highly non-progressive’ (≥+5) to assess characteristics of those with faster GFR decline. A decline/increase of more than 5 ml/min/1.73 m2/year during average follow-up represents a reduction/improvement of ∼50% in GFR from the median of 30 ml/min/1.73 m2 at baseline.
The slope analysis was repeated in the 331 patients with 10 or more GFR measures before [median number 20 (IQR 14–32)] and after the referral [median number 24 (IQR 16–37)] to test if the rates of decline were affected by the number of valid measures. The linearity of the individual GFR slopes was assessed and accepted as an adequate description of the rate of GFR decline.
The majority of the remaining 221 patients, from the total 947 new referrals, who were excluded were missing a pre-referral slope (n = 150) due to less than three pre-referral serum creatinine measures. Forty-six patients were missing a post-referral slope due to death in 26, progression to RRT in two and moving from the area in six patients. A further 25 patients had insufficient pre- and post-referral serum creatinine measures. There was no significant difference in those with and without GFR decline slopes other than the number of deaths which was 211 (29%) in the case of those with valid pre- and post-referral GFR slopes, 35 (23%) in those with no pre-referral slopes and 26 (57%, P < 0.001) in those with no post-referral slopes.
Statistics
Standard descriptives of the group included numbers and percentages and means and SDs except baseline GFR and rates of GFR decline as medians and IQRs that best described the distributions. The comparison of characteristics between groups used t-test, Chi-squared and non-parametric tests. The paired t-test assessed differences in variables over time.
Binary variables of non-progressive (>−1 ml/min/1.73 m2/year) and progressive rates of GFR decline (≤−1 ml/min/1.73 m2/year) pre- and post-referral were created.
A univariate and multivariate logistic regression analysis tested whether age, sex, diabetic nephropathy, vascular disease, pre-referral GFR slope, and GFR, proteinuria, SBP and DBP at and 1 year after referral, and ACEI or angiotensin II receptor blocker (ARB) therapy at referral predicted a non-progressive GFR decline post referral.
A Cox proportional-hazard model was then used to assess how the risk of death differed in those with a non-progressive compared with a progressive GFR decline after referral. Factors associated with adverse outcomes were entered into a univariate model, including: age, sex, diabetic nephropathy, prior vascular disease, level of GFR, proteinuria, SBP and DBP and ACEI/ARB therapy at referral; and a progressive vs non-progressive rate of pre- and post-referral GFR decline.
A stepwise multivariate model then tested whether any effect of a slow GFR decline post-referral on survival remained constant after adjusting for obvious confounders in the following order:
Age and gender.
Age, gender, prior vascular disease and diabetic nephropathy.
Age, gender, prior vascular disease, diabetic nephropathy, baseline GFR and level of proteinuria and pre-referral GFR decline.
Age, gender, prior vascular disease, diabetic nephropathy, baseline GFR and level of proteinuria, pre-referral GFR decline, baseline serum haemoglobin and albumin level and use of ACEI/ARBs at referral.
Baseline GFR was used in the regression and Cox's models to control for the effect of decline from different levels of GFR. For example, the impact of declining from a GFR of 30 to 20 may be more than that from 40 to 30.
Values shown are hazard ratios, 95% confidence intervals (95% CI) and corresponding P values. The proportional hazards assumption was checked using log-minus-log plots and accepted as not violated.
All analyses were carried out using SPSS V12.0.
Results
At the first referral, the patients were elderly and predominantly male (Table 1). More than half had advanced CKD (stage 4–5) and 232 (32%) had >1 g/day of proteinuria. Relatively few patients met the UK Renal Association guidelines for systolic [182 (25%)] and diastolic [232 (32%)] BP, whilst more than two-thirds had recommended levels of serum haemoglobin [559 (77%)], albumin [561 (77%)], calcium [552 (76%)] and phosphate [661 (91%)]. Diabetic nephropathy and hypertension was the cause of CKD in 17 and 10% of cases, respectively, and in 13% the cause was unknown. Prior vascular disease was common and despite this, the use of ACEIs or ARBs, aspirin and anti-lipidaemics at referral was relatively low.
Baseline characteristics of all 726 patients
| Characteristics . | Value . |
|---|---|
| Age | 72 (14) |
| Male | 446 (61%) |
| Baseline MDRD GFR (ml/min/1.73 m2) Median (IQR) | 29 (18–38) |
| KDOQI Stage of CKD | |
| Stage 3 | 337 (46%) |
| Stage 4 | 251 (35%) |
| Stage 5 | 138 (19%) |
| Baseline proteinuria >1 g/day | 232 (32%) |
| Vascular disease at baseline | 320 (44%) |
| Diagnosis | |
| Unknown | 97 (13%) |
| Diabetic nephropathy | 124 (17%) |
| Polycystic kidney disease | 24 (3%) |
| Hypertensive nephropathy | 69 (10%) |
| Glomerulonephritis | 52 (7%) |
| Pyelo/interstitial nephritis | 62 (9%) |
| Myeloma/amyloid | 11 (2%) |
| Other | 237 (32%) |
| Reno-vascular/ischaemic | 50 (7%) |
| Systolic BP at referral (mmHg) | 155 (28) |
| Diastolic BP at referral (mmHg) | 84 (14) |
| Haemoglobin at referral (g/dl) | 115 (21) |
| Albumin at referral (g/l) | 34 (6) |
| Phosphate at referral (mmol/l) | 1.28 (0.46) |
| Calcium at referral (mmol/l) | 2.33 (0.19) |
| ACEI or ARB at baseline | 278 (38%) |
| Anti-lipidaemic agent at baseline | 135 (19%) |
| Aspirin at baseline | 256 (35%) |
| Characteristics . | Value . |
|---|---|
| Age | 72 (14) |
| Male | 446 (61%) |
| Baseline MDRD GFR (ml/min/1.73 m2) Median (IQR) | 29 (18–38) |
| KDOQI Stage of CKD | |
| Stage 3 | 337 (46%) |
| Stage 4 | 251 (35%) |
| Stage 5 | 138 (19%) |
| Baseline proteinuria >1 g/day | 232 (32%) |
| Vascular disease at baseline | 320 (44%) |
| Diagnosis | |
| Unknown | 97 (13%) |
| Diabetic nephropathy | 124 (17%) |
| Polycystic kidney disease | 24 (3%) |
| Hypertensive nephropathy | 69 (10%) |
| Glomerulonephritis | 52 (7%) |
| Pyelo/interstitial nephritis | 62 (9%) |
| Myeloma/amyloid | 11 (2%) |
| Other | 237 (32%) |
| Reno-vascular/ischaemic | 50 (7%) |
| Systolic BP at referral (mmHg) | 155 (28) |
| Diastolic BP at referral (mmHg) | 84 (14) |
| Haemoglobin at referral (g/dl) | 115 (21) |
| Albumin at referral (g/l) | 34 (6) |
| Phosphate at referral (mmol/l) | 1.28 (0.46) |
| Calcium at referral (mmol/l) | 2.33 (0.19) |
| ACEI or ARB at baseline | 278 (38%) |
| Anti-lipidaemic agent at baseline | 135 (19%) |
| Aspirin at baseline | 256 (35%) |
Values are means and standard deviations or numbers and percentages unless stated.
Baseline characteristics of all 726 patients
| Characteristics . | Value . |
|---|---|
| Age | 72 (14) |
| Male | 446 (61%) |
| Baseline MDRD GFR (ml/min/1.73 m2) Median (IQR) | 29 (18–38) |
| KDOQI Stage of CKD | |
| Stage 3 | 337 (46%) |
| Stage 4 | 251 (35%) |
| Stage 5 | 138 (19%) |
| Baseline proteinuria >1 g/day | 232 (32%) |
| Vascular disease at baseline | 320 (44%) |
| Diagnosis | |
| Unknown | 97 (13%) |
| Diabetic nephropathy | 124 (17%) |
| Polycystic kidney disease | 24 (3%) |
| Hypertensive nephropathy | 69 (10%) |
| Glomerulonephritis | 52 (7%) |
| Pyelo/interstitial nephritis | 62 (9%) |
| Myeloma/amyloid | 11 (2%) |
| Other | 237 (32%) |
| Reno-vascular/ischaemic | 50 (7%) |
| Systolic BP at referral (mmHg) | 155 (28) |
| Diastolic BP at referral (mmHg) | 84 (14) |
| Haemoglobin at referral (g/dl) | 115 (21) |
| Albumin at referral (g/l) | 34 (6) |
| Phosphate at referral (mmol/l) | 1.28 (0.46) |
| Calcium at referral (mmol/l) | 2.33 (0.19) |
| ACEI or ARB at baseline | 278 (38%) |
| Anti-lipidaemic agent at baseline | 135 (19%) |
| Aspirin at baseline | 256 (35%) |
| Characteristics . | Value . |
|---|---|
| Age | 72 (14) |
| Male | 446 (61%) |
| Baseline MDRD GFR (ml/min/1.73 m2) Median (IQR) | 29 (18–38) |
| KDOQI Stage of CKD | |
| Stage 3 | 337 (46%) |
| Stage 4 | 251 (35%) |
| Stage 5 | 138 (19%) |
| Baseline proteinuria >1 g/day | 232 (32%) |
| Vascular disease at baseline | 320 (44%) |
| Diagnosis | |
| Unknown | 97 (13%) |
| Diabetic nephropathy | 124 (17%) |
| Polycystic kidney disease | 24 (3%) |
| Hypertensive nephropathy | 69 (10%) |
| Glomerulonephritis | 52 (7%) |
| Pyelo/interstitial nephritis | 62 (9%) |
| Myeloma/amyloid | 11 (2%) |
| Other | 237 (32%) |
| Reno-vascular/ischaemic | 50 (7%) |
| Systolic BP at referral (mmHg) | 155 (28) |
| Diastolic BP at referral (mmHg) | 84 (14) |
| Haemoglobin at referral (g/dl) | 115 (21) |
| Albumin at referral (g/l) | 34 (6) |
| Phosphate at referral (mmol/l) | 1.28 (0.46) |
| Calcium at referral (mmol/l) | 2.33 (0.19) |
| ACEI or ARB at baseline | 278 (38%) |
| Anti-lipidaemic agent at baseline | 135 (19%) |
| Aspirin at baseline | 256 (35%) |
Values are means and standard deviations or numbers and percentages unless stated.
Rates of pre- and post-referral GFR decline
The median (IQR) rate of GFR decline slowed significantly from −5.4(−13 to −2) ml/min/1.73 m2/year before referral to −0.35(−3 to +3) ml/min/1.73 m2/year after referral (P < 0.001) in the 726 patients with valid slopes.
In the 331 patients with more than 10 GFR measures pre- and post-referral, the decline changed from −6.3(−14 to −3) ml/min/1.73 m2/year to −0.04(−4 to +4) ml/min/1.73 m2/year (P < 0.001).
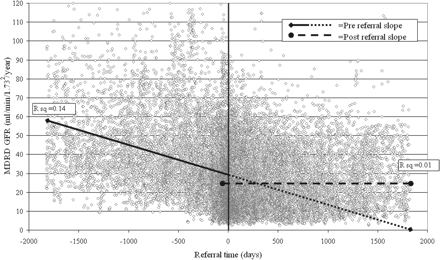
Extrapolation of the regression line of pre-referral GFR decline suggests that if the same rate continued after the referral, on average the absolute GFR would fall below 10 ml/minute/1.73 m2 within 4 years of the referral (Figure 1). The point of intercept of the pre- and post-referral regression lines is at approximately 9 months after referral and indicates on average the time at which the rate of progression changes to a slower decline.
Decline in MDRD GFR in the 5 years prior to and following nephrology referral with regression lines of summary pre- and post-referral GFR slopes (each point is a single GFR measure).
Changes in the rates of GFR decline prior to and following referral
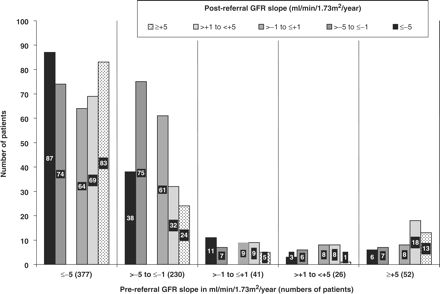
Prior to referral, 607(84%) patients had a progressive GFR decline ≤−1 ml/min/1.73 m2/year, and 377 (52%) of these patients had a fast decline ≤−5 ml/min/1.73 m2/year (Figure 2). After referral, in 333 (55%) patients the decline slowed to >−1 ml/min/1.73 m2/year, and in 274 (45%) the progressive rate continued. Of the 119 (16%) patients with a non-progressive rate of GFR decline prior to referral, 40 (34%) increased to a progressive decline after referral, 20 (17%) of which to a fast rate of ≤−5 ml/min/1.73 m2/year.
Numbers of patients in categories of post-referral GFR slope by their category of pre-referral GFR slope.
Characteristics of the post-referral progressors and non-progressors
The post-referral progressors had a significantly faster rate of pre-referral GFR decline, a higher level of proteinuria and SBP at referral and more diabetic nephropathy (Table 2) than non-progressors. The fast progressors had a significantly faster rate of pre-referral GFR decline, higher levels of proteinuria at referral and lower serum haemoglobin levels than slow progressors.
Characteristics of those with progressive and non-progressive rates of post-referral GFR decline (ml/min/1.73 m2/year)
| Characteristics . | Progressive slope (n = 314) . | . | . | Non-progressive slope (n = 412) . | Total (n = 726) . | ||
|---|---|---|---|---|---|---|---|
| . | ≤−5 ml/min/year 145 (46%) . | >−5 to ≤−1 ml/min/year 169 (54%) . | All progressive 314 (100%) . | . | . | ||
| Age in years | 69 (17) | 73 (12) | 71 (14) | 72 (14) | 72(14) | ||
| Sex (male) | 88(61%) | 97(57%) | 185 (59%) | 261 (63%) | 446(61%) | ||
| Pre-referral GFR decline Median (IQR) | −6.8† (−14 to −3) | −4.4 (−8 to −2) | −5.3 (−11 to −2) | −5.4# (−14 to −2) | −5.4 (−13 to −2) | ||
| GFR at referral Median (IQR) | 27 (14-36) | 31 (21–40) | 29 (18–39) | 28 (18–37) | 29(18–38) | ||
| Proteinuria >1 g/day | 67 (46%)† | 51 (30%) | 118 (38%) | 114 (28%)# | 232 (32%) | ||
| Systolic BP at referral (mmHg) | 157 (31) | 158 (25) | 158 (28) | 153 (28)# | 155 (28) | ||
| Diastolic BP at referral (mmHg) | 83 (15) | 85 (13) | 84 (14) | 83 (15) | 84 (14) | ||
| Haemoglobin at referral (g/l) | 110 (22)† | 118 (21) | 114 (22) | 116 (21) | 115 (21) | ||
| Albumin (g/l) | 32 (7) | 36 (5) | 34 (6) | 34 (7) | 34 (6) | ||
| Diabetic nephropathy | 32 (22%) | 32 (19%) | 64 (21%) | 60 (15%)# | 124 (17%) | ||
| Vascular disease | 61 (42%) | 77 (46%) | 138 (44%) | 182 (44%) | 320 (44%) | ||
| ACEI/ARB therapy at referral | 64 (44%) | 61 (36%) | 125 (40%) | 153 (37%) | 278 (38%) | ||
| Anti-lipid therapy at referral | 26 (18%) | 35 (21%) | 61 (19%) | 74 (18%) | 135 (19%) | ||
| Aspirin therapy at referral | 44 (30%) | 62 (37%) | 106 (34%) | 150 (36%) | 256 (35%) | ||
| Characteristics . | Progressive slope (n = 314) . | . | . | Non-progressive slope (n = 412) . | Total (n = 726) . | ||
|---|---|---|---|---|---|---|---|
| . | ≤−5 ml/min/year 145 (46%) . | >−5 to ≤−1 ml/min/year 169 (54%) . | All progressive 314 (100%) . | . | . | ||
| Age in years | 69 (17) | 73 (12) | 71 (14) | 72 (14) | 72(14) | ||
| Sex (male) | 88(61%) | 97(57%) | 185 (59%) | 261 (63%) | 446(61%) | ||
| Pre-referral GFR decline Median (IQR) | −6.8† (−14 to −3) | −4.4 (−8 to −2) | −5.3 (−11 to −2) | −5.4# (−14 to −2) | −5.4 (−13 to −2) | ||
| GFR at referral Median (IQR) | 27 (14-36) | 31 (21–40) | 29 (18–39) | 28 (18–37) | 29(18–38) | ||
| Proteinuria >1 g/day | 67 (46%)† | 51 (30%) | 118 (38%) | 114 (28%)# | 232 (32%) | ||
| Systolic BP at referral (mmHg) | 157 (31) | 158 (25) | 158 (28) | 153 (28)# | 155 (28) | ||
| Diastolic BP at referral (mmHg) | 83 (15) | 85 (13) | 84 (14) | 83 (15) | 84 (14) | ||
| Haemoglobin at referral (g/l) | 110 (22)† | 118 (21) | 114 (22) | 116 (21) | 115 (21) | ||
| Albumin (g/l) | 32 (7) | 36 (5) | 34 (6) | 34 (7) | 34 (6) | ||
| Diabetic nephropathy | 32 (22%) | 32 (19%) | 64 (21%) | 60 (15%)# | 124 (17%) | ||
| Vascular disease | 61 (42%) | 77 (46%) | 138 (44%) | 182 (44%) | 320 (44%) | ||
| ACEI/ARB therapy at referral | 64 (44%) | 61 (36%) | 125 (40%) | 153 (37%) | 278 (38%) | ||
| Anti-lipid therapy at referral | 26 (18%) | 35 (21%) | 61 (19%) | 74 (18%) | 135 (19%) | ||
| Aspirin therapy at referral | 44 (30%) | 62 (37%) | 106 (34%) | 150 (36%) | 256 (35%) | ||
Values are numbers and percentages or means and SDs unless stated.
#P < 0.05 comparing all 314 post-referral progressors (≤−1ml/min/year) and the 412 non-progressors (>−1 ml/min/year). †P < 0.05 comparing the 145 fast post-referal progressors (≤−5 ml/min/year) and the 169 slow progressors (>−5 to ≤−1 ml/min/year).
Characteristics of those with progressive and non-progressive rates of post-referral GFR decline (ml/min/1.73 m2/year)
| Characteristics . | Progressive slope (n = 314) . | . | . | Non-progressive slope (n = 412) . | Total (n = 726) . | ||
|---|---|---|---|---|---|---|---|
| . | ≤−5 ml/min/year 145 (46%) . | >−5 to ≤−1 ml/min/year 169 (54%) . | All progressive 314 (100%) . | . | . | ||
| Age in years | 69 (17) | 73 (12) | 71 (14) | 72 (14) | 72(14) | ||
| Sex (male) | 88(61%) | 97(57%) | 185 (59%) | 261 (63%) | 446(61%) | ||
| Pre-referral GFR decline Median (IQR) | −6.8† (−14 to −3) | −4.4 (−8 to −2) | −5.3 (−11 to −2) | −5.4# (−14 to −2) | −5.4 (−13 to −2) | ||
| GFR at referral Median (IQR) | 27 (14-36) | 31 (21–40) | 29 (18–39) | 28 (18–37) | 29(18–38) | ||
| Proteinuria >1 g/day | 67 (46%)† | 51 (30%) | 118 (38%) | 114 (28%)# | 232 (32%) | ||
| Systolic BP at referral (mmHg) | 157 (31) | 158 (25) | 158 (28) | 153 (28)# | 155 (28) | ||
| Diastolic BP at referral (mmHg) | 83 (15) | 85 (13) | 84 (14) | 83 (15) | 84 (14) | ||
| Haemoglobin at referral (g/l) | 110 (22)† | 118 (21) | 114 (22) | 116 (21) | 115 (21) | ||
| Albumin (g/l) | 32 (7) | 36 (5) | 34 (6) | 34 (7) | 34 (6) | ||
| Diabetic nephropathy | 32 (22%) | 32 (19%) | 64 (21%) | 60 (15%)# | 124 (17%) | ||
| Vascular disease | 61 (42%) | 77 (46%) | 138 (44%) | 182 (44%) | 320 (44%) | ||
| ACEI/ARB therapy at referral | 64 (44%) | 61 (36%) | 125 (40%) | 153 (37%) | 278 (38%) | ||
| Anti-lipid therapy at referral | 26 (18%) | 35 (21%) | 61 (19%) | 74 (18%) | 135 (19%) | ||
| Aspirin therapy at referral | 44 (30%) | 62 (37%) | 106 (34%) | 150 (36%) | 256 (35%) | ||
| Characteristics . | Progressive slope (n = 314) . | . | . | Non-progressive slope (n = 412) . | Total (n = 726) . | ||
|---|---|---|---|---|---|---|---|
| . | ≤−5 ml/min/year 145 (46%) . | >−5 to ≤−1 ml/min/year 169 (54%) . | All progressive 314 (100%) . | . | . | ||
| Age in years | 69 (17) | 73 (12) | 71 (14) | 72 (14) | 72(14) | ||
| Sex (male) | 88(61%) | 97(57%) | 185 (59%) | 261 (63%) | 446(61%) | ||
| Pre-referral GFR decline Median (IQR) | −6.8† (−14 to −3) | −4.4 (−8 to −2) | −5.3 (−11 to −2) | −5.4# (−14 to −2) | −5.4 (−13 to −2) | ||
| GFR at referral Median (IQR) | 27 (14-36) | 31 (21–40) | 29 (18–39) | 28 (18–37) | 29(18–38) | ||
| Proteinuria >1 g/day | 67 (46%)† | 51 (30%) | 118 (38%) | 114 (28%)# | 232 (32%) | ||
| Systolic BP at referral (mmHg) | 157 (31) | 158 (25) | 158 (28) | 153 (28)# | 155 (28) | ||
| Diastolic BP at referral (mmHg) | 83 (15) | 85 (13) | 84 (14) | 83 (15) | 84 (14) | ||
| Haemoglobin at referral (g/l) | 110 (22)† | 118 (21) | 114 (22) | 116 (21) | 115 (21) | ||
| Albumin (g/l) | 32 (7) | 36 (5) | 34 (6) | 34 (7) | 34 (6) | ||
| Diabetic nephropathy | 32 (22%) | 32 (19%) | 64 (21%) | 60 (15%)# | 124 (17%) | ||
| Vascular disease | 61 (42%) | 77 (46%) | 138 (44%) | 182 (44%) | 320 (44%) | ||
| ACEI/ARB therapy at referral | 64 (44%) | 61 (36%) | 125 (40%) | 153 (37%) | 278 (38%) | ||
| Anti-lipid therapy at referral | 26 (18%) | 35 (21%) | 61 (19%) | 74 (18%) | 135 (19%) | ||
| Aspirin therapy at referral | 44 (30%) | 62 (37%) | 106 (34%) | 150 (36%) | 256 (35%) | ||
Values are numbers and percentages or means and SDs unless stated.
#P < 0.05 comparing all 314 post-referral progressors (≤−1ml/min/year) and the 412 non-progressors (>−1 ml/min/year). †P < 0.05 comparing the 145 fast post-referal progressors (≤−5 ml/min/year) and the 169 slow progressors (>−5 to ≤−1 ml/min/year).
A total of 278 (38%) patients were prescribed an ACEI/ARB at referral, with a further 75 (12%) prescribed at 1 year and 30 (5%) at 3 years after referral in the 621 and 548 patients still alive. No significant difference was seen in the use of ACEI/ARB between the groups at referral, 1 or 3 years. Anti-lipidaemic therapy use at 1 and 3 years increased by 42 (7%) and 13 (2%) and aspirin prescribing by 11 (2%) and eight (2%) patients, though again the difference between the groups was not significant. This highlights that the majority of changes in medications occur within the first year of referral. Erythropoeitin use was low in all groups with 54 (7%) of all patients prescribed at 3 years.
Changes in blood pressure
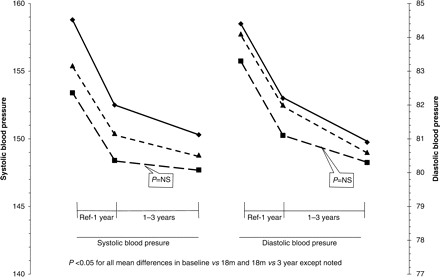
Figure 3 shows that the majority of BP reduction occurred within the first year of referral. In all patients, non-progressors and progressors with valid measures, the SBPs and DBPs fell significantly between referral and 1 year, and 1 and 3 years (P < 0.05) except in the ‘non-progressors’ in the latter period. The SBP was significantly lower in the non-progressors than in the progressors at referral and 1 year (P < 0.05), though not at 3 years or in DBP at any of the time points.
Change in SBP and DBP between referral and 1 year post-referral (Ref-1 year), and between 1 and 3 years post-referral (1–3 years). Includes all patients (n = 652), and post-referral ‘non-progressors’ (n = 369) and ‘progressors’ (n = 283) with valid BP measures at all time points. All patients (closed triangle) ‘non-progressors’ (closed square) and ‘progressors’ (closed diamond).
Factors predicting a non-progressive post-referral GFR decline
A diagnosis of diabetic nephropathy, baseline proteinuria >1 g/day and a progressive pre-referral GFR decline significantly reduced the likelihood of a non-progressive post-referral GFR decline by 33, 38 and 38%, respectively, whilst a 10 mmHg higher SBP at referral and 1 year after were both associated with a 10% lower likelihood (assuming the relationship is linear) (Table 3). Following mutual adjustment in a multivariate model, only baseline proteinuria >1 g/day (hazard ratio 0.63, 95% CI 0.43–0.91, P = 0.01) and the rate of pre-referral GFR decline remained significantly associated with a non-progressive post-referral decline (hazard ratio 0.54, 95% CI 0.30–0.98, P = 0.04).
Univariate factors predicting a non-progressive post-referral GFR slope in all 726 patients
| Characteristics . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Age at referral (per year increase) | 1.05 | 0.99–1.02 | 0.32 |
| Female sex | 0.83 | 0.61–1.12 | 0.22 |
| Diabetic nephropathy | 0.67 | 0.0.45–0.98 | 0.04 |
| Vascular disease | 1.01 | 0.75–1.36 | 0.95 |
| Progressivevsnon-progressive pre-referral GFR decline | 0.62 | 0.41–0.93 | 0.02 |
| MDRD GFR at referral | 0.99 | 0.99–0.01 | 0.67 |
| Proteinuria at referral (vs <300 mg/day) | |||
| 300 mg–1 g/day | 0.88 | 0.56–0.39 | 0.59 |
| >1 g/day | 0.62 | 0.45–0.86 | 0.004 |
| SBP at referral (per mmHg increase) | 0.99 | 0.98–0.99 | 0.03 |
| SBP at 1 year (per mmHg increase) | 0.99 | 0.98–0.99 | 0.02 |
| DBP at referral (per mmHg increase) | 0.99 | 0.98–1.01 | 0.37 |
| DBP at 1 year (per mmHg increase) | 0.98 | 0.97–1.00 | 0.13 |
| ACEI/ARB therapy at referral | 1.14 | 0.85–1.53 | 0.37 |
| Characteristics . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Age at referral (per year increase) | 1.05 | 0.99–1.02 | 0.32 |
| Female sex | 0.83 | 0.61–1.12 | 0.22 |
| Diabetic nephropathy | 0.67 | 0.0.45–0.98 | 0.04 |
| Vascular disease | 1.01 | 0.75–1.36 | 0.95 |
| Progressivevsnon-progressive pre-referral GFR decline | 0.62 | 0.41–0.93 | 0.02 |
| MDRD GFR at referral | 0.99 | 0.99–0.01 | 0.67 |
| Proteinuria at referral (vs <300 mg/day) | |||
| 300 mg–1 g/day | 0.88 | 0.56–0.39 | 0.59 |
| >1 g/day | 0.62 | 0.45–0.86 | 0.004 |
| SBP at referral (per mmHg increase) | 0.99 | 0.98–0.99 | 0.03 |
| SBP at 1 year (per mmHg increase) | 0.99 | 0.98–0.99 | 0.02 |
| DBP at referral (per mmHg increase) | 0.99 | 0.98–1.01 | 0.37 |
| DBP at 1 year (per mmHg increase) | 0.98 | 0.97–1.00 | 0.13 |
| ACEI/ARB therapy at referral | 1.14 | 0.85–1.53 | 0.37 |
Univariate factors predicting a non-progressive post-referral GFR slope in all 726 patients
| Characteristics . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Age at referral (per year increase) | 1.05 | 0.99–1.02 | 0.32 |
| Female sex | 0.83 | 0.61–1.12 | 0.22 |
| Diabetic nephropathy | 0.67 | 0.0.45–0.98 | 0.04 |
| Vascular disease | 1.01 | 0.75–1.36 | 0.95 |
| Progressivevsnon-progressive pre-referral GFR decline | 0.62 | 0.41–0.93 | 0.02 |
| MDRD GFR at referral | 0.99 | 0.99–0.01 | 0.67 |
| Proteinuria at referral (vs <300 mg/day) | |||
| 300 mg–1 g/day | 0.88 | 0.56–0.39 | 0.59 |
| >1 g/day | 0.62 | 0.45–0.86 | 0.004 |
| SBP at referral (per mmHg increase) | 0.99 | 0.98–0.99 | 0.03 |
| SBP at 1 year (per mmHg increase) | 0.99 | 0.98–0.99 | 0.02 |
| DBP at referral (per mmHg increase) | 0.99 | 0.98–1.01 | 0.37 |
| DBP at 1 year (per mmHg increase) | 0.98 | 0.97–1.00 | 0.13 |
| ACEI/ARB therapy at referral | 1.14 | 0.85–1.53 | 0.37 |
| Characteristics . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Age at referral (per year increase) | 1.05 | 0.99–1.02 | 0.32 |
| Female sex | 0.83 | 0.61–1.12 | 0.22 |
| Diabetic nephropathy | 0.67 | 0.0.45–0.98 | 0.04 |
| Vascular disease | 1.01 | 0.75–1.36 | 0.95 |
| Progressivevsnon-progressive pre-referral GFR decline | 0.62 | 0.41–0.93 | 0.02 |
| MDRD GFR at referral | 0.99 | 0.99–0.01 | 0.67 |
| Proteinuria at referral (vs <300 mg/day) | |||
| 300 mg–1 g/day | 0.88 | 0.56–0.39 | 0.59 |
| >1 g/day | 0.62 | 0.45–0.86 | 0.004 |
| SBP at referral (per mmHg increase) | 0.99 | 0.98–0.99 | 0.03 |
| SBP at 1 year (per mmHg increase) | 0.99 | 0.98–0.99 | 0.02 |
| DBP at referral (per mmHg increase) | 0.99 | 0.98–1.01 | 0.37 |
| DBP at 1 year (per mmHg increase) | 0.98 | 0.97–1.00 | 0.13 |
| ACEI/ARB therapy at referral | 1.14 | 0.85–1.53 | 0.37 |
Survival
A total of 105 (15%) patients died within 1 year and 178 (25%) within 3 years of referral. For seventy-three (10%) of the patients, RRT was started within 3 years of referral.
The univariate Cox model (Table 4) showed that a slower rate of GFR decline post-referral was associated with a lower risk of death, as was a higher baseline GFR, serum haemoglobin and albumin level and ACEI or ARB therapy at referral. It also showed that increasing age, prior vascular disease, a progressive pre-referral GFR decline, and a higher level of proteinuria at referral significantly increased the risk of death.
Univariate Cox Proportional Hazard model of factors predicting death in all 726 patients
| . | Hazard ratio . |
|---|---|
| Age at referral (per year increase) | 1.05† (1.03–1.06) |
| Female sex | 0.81 (0.61–1.08) |
| Diabetic nephropathy | 1.26 (0.89–1.79) |
| Vascular disease | 1.51‡ (1.15–1.97) |
| MDRD GFR at referral | 0.96† (0.95–0.98) |
| Progressive vs non-progressive pre-referral GFR decline | 1.66# (1.10–2.51) |
| Proteinuria at referral (vs <300 mg/day) | |
| 300 mg–1 g/day | 1.02 (0.66–1.60) |
| >1 g/day | 1.99† (1.50–2.66) |
| SBP at referral (per mmHg increase) | 1.00 (0.99–1.01) |
| DBP at referral (per mmHg increase) | 0.99 (0.97–1.00) |
| Haemoglobin at referral (per g/dl increase) | 0.98† (0.97–0.98) |
| Albumin at referral (per g/l increase) | 0.92† (0.91–0.94) |
| ACEI/ARB therapy | 0.68‡ (0.52–0.90) |
| Non-progressive vs progressive post-referral GFR decline | 0.61† (0.47–0.80) |
| . | Hazard ratio . |
|---|---|
| Age at referral (per year increase) | 1.05† (1.03–1.06) |
| Female sex | 0.81 (0.61–1.08) |
| Diabetic nephropathy | 1.26 (0.89–1.79) |
| Vascular disease | 1.51‡ (1.15–1.97) |
| MDRD GFR at referral | 0.96† (0.95–0.98) |
| Progressive vs non-progressive pre-referral GFR decline | 1.66# (1.10–2.51) |
| Proteinuria at referral (vs <300 mg/day) | |
| 300 mg–1 g/day | 1.02 (0.66–1.60) |
| >1 g/day | 1.99† (1.50–2.66) |
| SBP at referral (per mmHg increase) | 1.00 (0.99–1.01) |
| DBP at referral (per mmHg increase) | 0.99 (0.97–1.00) |
| Haemoglobin at referral (per g/dl increase) | 0.98† (0.97–0.98) |
| Albumin at referral (per g/l increase) | 0.92† (0.91–0.94) |
| ACEI/ARB therapy | 0.68‡ (0.52–0.90) |
| Non-progressive vs progressive post-referral GFR decline | 0.61† (0.47–0.80) |
Values are hazard ratios and 95% CI.
#P < 0.05; ‡P < 0.01; †P < 0.001.
Univariate Cox Proportional Hazard model of factors predicting death in all 726 patients
| . | Hazard ratio . |
|---|---|
| Age at referral (per year increase) | 1.05† (1.03–1.06) |
| Female sex | 0.81 (0.61–1.08) |
| Diabetic nephropathy | 1.26 (0.89–1.79) |
| Vascular disease | 1.51‡ (1.15–1.97) |
| MDRD GFR at referral | 0.96† (0.95–0.98) |
| Progressive vs non-progressive pre-referral GFR decline | 1.66# (1.10–2.51) |
| Proteinuria at referral (vs <300 mg/day) | |
| 300 mg–1 g/day | 1.02 (0.66–1.60) |
| >1 g/day | 1.99† (1.50–2.66) |
| SBP at referral (per mmHg increase) | 1.00 (0.99–1.01) |
| DBP at referral (per mmHg increase) | 0.99 (0.97–1.00) |
| Haemoglobin at referral (per g/dl increase) | 0.98† (0.97–0.98) |
| Albumin at referral (per g/l increase) | 0.92† (0.91–0.94) |
| ACEI/ARB therapy | 0.68‡ (0.52–0.90) |
| Non-progressive vs progressive post-referral GFR decline | 0.61† (0.47–0.80) |
| . | Hazard ratio . |
|---|---|
| Age at referral (per year increase) | 1.05† (1.03–1.06) |
| Female sex | 0.81 (0.61–1.08) |
| Diabetic nephropathy | 1.26 (0.89–1.79) |
| Vascular disease | 1.51‡ (1.15–1.97) |
| MDRD GFR at referral | 0.96† (0.95–0.98) |
| Progressive vs non-progressive pre-referral GFR decline | 1.66# (1.10–2.51) |
| Proteinuria at referral (vs <300 mg/day) | |
| 300 mg–1 g/day | 1.02 (0.66–1.60) |
| >1 g/day | 1.99† (1.50–2.66) |
| SBP at referral (per mmHg increase) | 1.00 (0.99–1.01) |
| DBP at referral (per mmHg increase) | 0.99 (0.97–1.00) |
| Haemoglobin at referral (per g/dl increase) | 0.98† (0.97–0.98) |
| Albumin at referral (per g/l increase) | 0.92† (0.91–0.94) |
| ACEI/ARB therapy | 0.68‡ (0.52–0.90) |
| Non-progressive vs progressive post-referral GFR decline | 0.61† (0.47–0.80) |
Values are hazard ratios and 95% CI.
#P < 0.05; ‡P < 0.01; †P < 0.001.
In a stepwise multivariate Cox regression analysis, a non-progressive GFR decline post referral was associated with a 45% lower risk of death than a progressive post-referral GFR decline (Table 5).
Multivariate stepwise Cox proportional hazard model of the association of non-progressive post-referral GFR decline with survival in all 726 patients
| Characteristics . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Non-progressive vs progressive post-referral GFR decline | 0.61 | 0.47–0.80 | <0.001 |
| + Age and sex | 0.57 | 0.43–0.75 | <0.001 |
| + Age, sex, diabetic nephropathy and prior vascular disease | 0.58 | 0.44–0.76 | <0.001 |
| + Age, sex, diabetic nephropathy, prior vascular disease, pre-referral GFR slope and baseline GFR and proteinuria | 0.59 | 0.45–0.79 | <0.001 |
| + Age, sex, diabetic nephropathy, prior vascular disease, pre-referral GFR slope, baseline GFR and proteinuria, serum haemoglobin and albumin and ACEI/ARB therapy at referral | 0.55 | 0.40–0.75 | <0.001 |
| Characteristics . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Non-progressive vs progressive post-referral GFR decline | 0.61 | 0.47–0.80 | <0.001 |
| + Age and sex | 0.57 | 0.43–0.75 | <0.001 |
| + Age, sex, diabetic nephropathy and prior vascular disease | 0.58 | 0.44–0.76 | <0.001 |
| + Age, sex, diabetic nephropathy, prior vascular disease, pre-referral GFR slope and baseline GFR and proteinuria | 0.59 | 0.45–0.79 | <0.001 |
| + Age, sex, diabetic nephropathy, prior vascular disease, pre-referral GFR slope, baseline GFR and proteinuria, serum haemoglobin and albumin and ACEI/ARB therapy at referral | 0.55 | 0.40–0.75 | <0.001 |
Values are hazard ratios, 95% CIs and P-values.
Multivariate stepwise Cox proportional hazard model of the association of non-progressive post-referral GFR decline with survival in all 726 patients
| Characteristics . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Non-progressive vs progressive post-referral GFR decline | 0.61 | 0.47–0.80 | <0.001 |
| + Age and sex | 0.57 | 0.43–0.75 | <0.001 |
| + Age, sex, diabetic nephropathy and prior vascular disease | 0.58 | 0.44–0.76 | <0.001 |
| + Age, sex, diabetic nephropathy, prior vascular disease, pre-referral GFR slope and baseline GFR and proteinuria | 0.59 | 0.45–0.79 | <0.001 |
| + Age, sex, diabetic nephropathy, prior vascular disease, pre-referral GFR slope, baseline GFR and proteinuria, serum haemoglobin and albumin and ACEI/ARB therapy at referral | 0.55 | 0.40–0.75 | <0.001 |
| Characteristics . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Non-progressive vs progressive post-referral GFR decline | 0.61 | 0.47–0.80 | <0.001 |
| + Age and sex | 0.57 | 0.43–0.75 | <0.001 |
| + Age, sex, diabetic nephropathy and prior vascular disease | 0.58 | 0.44–0.76 | <0.001 |
| + Age, sex, diabetic nephropathy, prior vascular disease, pre-referral GFR slope and baseline GFR and proteinuria | 0.59 | 0.45–0.79 | <0.001 |
| + Age, sex, diabetic nephropathy, prior vascular disease, pre-referral GFR slope, baseline GFR and proteinuria, serum haemoglobin and albumin and ACEI/ARB therapy at referral | 0.55 | 0.40–0.75 | <0.001 |
Values are hazard ratios, 95% CIs and P-values.
Discussion
In this single nephrology clinic study, GFR decline slowed significantly after referral, and was associated with significantly better survival following adjustment for many traditional risk factors. More than half of those with a progressive GFR decline prior to referral changed to a non-progressive slope after referral despite a substantial number having a decline faster than −5 ml/min/1.73 m2/year and advanced CKD. However, not all patients improved as some remained or changed to a progressive GFR decline. Whilst this study has not been able to prove when renal function stabilizes, the intercept of the GFR regression lines suggests that this happens on average at ∼9 months after nephrology referral.
This raises two main questions: why did the slowing of GFR decline occur and why is there an association with mortality?
GFR decline
The slowing of GFR decline could be due to differing serum creatinine testing methods before and after referral. However, 95% of tests were carried out in one laboratory and testing practices did not change during the time period of the study. An effect of regression on the mean cannot be discounted, however, GFR measures were relatively frequent and recorded over two long time periods, increasing the validity of estimates of decline [28]. Also, the number of GFR measures did not appear to influence the change in rates as very similar results were obtained in those with more than 10 measures pre- and post-referral.
Without calibration of this laboratory's serum creatinine tests to those of the MDRD study, the estimated GFR may be inaccurate [29]. This may lead to misclassification of cases into stages of CKD. It may also lead to an error in estimating pre- and post-referral GFR slopes if true GFR is higher than our estimates at lower levels of serum creatinine and lower than our estimates at higher levels of serum creatinine. Our laboratory has no method for standardizing serum creatinines with those of the MDRD Study laboratory and there is no single recognized correction factor one can apply since it is dependent on each laboratory's specific method [29–33]. We used the MDRD GFR formula as it is accepted as the more accurate of the available methods [31], especially in a group of patients such as ours with more advanced CKD [34].
The change in rate of decline we have observed may have been influenced by changes in service patterns. In the latter years of this study (1997–2003), the UK saw the implementation of the Coronary Heart Disease [35] and Diabetes [36] National Service Frameworks (NSFs) which detail quality requirements and markers of good practice, especially in primary care. However, the impact is likely to be small as the post-referral care of the patients in this study was predominantly by nephrologists.
Most factors associated with a faster post-referral GFR decline were non-modifiable in terms of nephrology interventions (diagnosis of diabetic nephropathy [37,38], SBP and proteinuria at referral [39–42] and pre-referral rate of GFR decline). However, a lower average SBP at 1 year also predicted a non-progressive post-referral decline which suggests that a good BP control early in the disease process is the key to slowing decline. The reduction in post-referral BPs could, of course, be partly due to a regression to the mean, or differences in BP measurement, although despite different observers and BP machines being used over time, this is unlikely to lead to a systematic fall in BP. It may also be due to more or different antihypertensive agents or better adherence. We had no data on the former, and whether referral to a specialist and the threat of established kidney failure encouraged the patients into closer adherence to their treatment regimes needs more research.
The effect of improved BP control on GFR decline is supported by an apparent change in GFR slope at 9 months and also the observed major reduction in BP within the first year of referral. This confirms the data from a recent post hoc analysis [43], showing mean follow-up SBP to be more strongly correlated with a doubling in serum creatinine or progression to ERF than SBP at referral. However, others [16,44] have found that only in those with higher levels of baseline proteinuria did a lower BP target predict a slower decline in renal function, with mean BP associated with progression once levels were above 112 mmHg [45].
Another mechanism by which GFR decline may be mediated is through an increase in prescribing of ACEI/ARBs [46–50]. However, in this study we found no association between baseline ACEI/ARB use and GFR decline, and whilst their use increased significantly after referral, GFR was still relatively low. Also, there was no difference in their use at baseline, 1 and 3 years after referral in those with and without a progressive GFR decline. We may have failed to detect an effect of these drugs at referral due to residual confounding as their prescription may be associated with factors predictive of progression such as proteinuria.
The use of low protein diets to slow the progression of CKD is not recommended in this unit and as such we do not expect this to have affected the rates of decline in GFR.
Others have previously shown the benefit of specialist renal clinics in slowing CKD progression [19,51], and whilst they were unable to prove it was due to BP control, lower BP has been associated with slower CKD progression [52–54]. Bergstrom et al. [51] studied in 17 patients, prior to entry to a clinical trial, the benefits of a low protein diet and found a significant slowing in the progression of CKD as measured by reciprocals of serum creatinine. In their study, a reduction in blood pressure was significantly correlated with a slowing in decline and the authors concluded that more frequent and possibly better-quality check-ups may have influenced the change in decline. In another study, Feest et al. [19] investigated the rate of GFR decline in patients with diabetic nephropathy before and after referral to a nephrology clinic. Since they also used reciprocal serum creatinine plots rather than rates of estimated GFR decline, direct comparisons of progression rates were not possible, but they did find that the renal function decline slowed after referral in a significant number of their subjects [(n = 30 (39%)]. Also, although they were unable to identify a single factor predictive of a slowing of decline, SBP but not DBP, fell significantly after referral to their clinic.
Mortality findings
The effect of GFR decline on survival could be a real effect or due to bias or confounding. The exclusion of cases with no post-referral GFR slopes, with a high mortality rate and higher than average pre-referral decline may underestimate the effect of GFR decline on mortality. The association maybe confounded by factors that affect both GFR decline and cardiovascular risk (such as BP control, ACEI/ARB use, change in weight and cessation of smoking), some of which we were unable to assess and it is possible that residual confounding is present. However, there is strong evidence of an association of GFR per se with cardiovascular and all-cause mortality risk [14,55,56], even though these have been based on single measures of kidney function. This study shows that irrespective of the absolute measure, a slower rate of GFR decline was associated with lower mortality, and supports others who found a lower risk of death in those with smaller increases in serum creatinine [57] and an increase in serum creatinine at 6 years to be predictive of all-cause mortality [58]. The latter were adjusted for age, DBP, body mass index, high and low-density lipoproteins, numbers of cigarettes smoked per day and baseline serum creatinine level. Other randomized trials have shown the benefit of specific interventions such as ACEIs in reducing CKD progression and the risk of death [46–50,59], though to our knowledge none has tested any independent effect of slowing decline on mortality.
Limitations
The key limitations of our study are its reliance on retrospective data from a single nephrology centre, the under-ascertainment of some important variables (smoking, dyslipidaemia, type and number of conventional antihypertensive agents) and the lack of a control group managed in a different setting. The Southampton area has a very small ethnic minority population and our results may not be generalizable to areas with more diverse populations. Follow-up was limited to an average of 3 years.
Implications
Our findings need confirming in other settings along with more research into the mechanisms of slowing of progression, including drug adherence. If a slowing in decline is associated with better patient survival, earlier identification and intervention for progressive CKD, for example, by more active surveillance of high risk groups would be beneficial. However, it is unclear whether this reduction in decline would have taken place without nephrology input. It has been suggested that as many interventions known to be beneficial in slowing CKD are recommended in other chronic illness areas [22], more CKDs may be managed in another setting with support from nephrologists. However, a significant number of patients continued to decline, and some changed to a progressive rate of decline after referral. It may be that at the very least, these patients with moderate to advanced CKD will need to have their care overseen by a nephrologist as complications of CKD will be more likely and there is a risk that the significance of a decline in GFR may not be recognized. A prospective trial randomizing patients to care in a nephrology clinic or other setting such as primary care would be needed to determine whether the positive changes we have observed would have occurred without nephrology intervention.
Conclusion
This study of moderate to advanced kidney disease from a single nephrology clinic shows that at referral, the rate of pre-referral GFR decline could be determined in the majority of cases allowing identification of those with a fast decline, which along with proteinuria was a strong predictor of post-referral decline and subsequent death. The earlier detection of patients with progressive CKD and interventions to slow progression may have benefits on both kidney and patient survival.
We are very grateful to the British Renal Society for providing a research grant to fund this work and to Mrs Natasha Wilson, the renal unit database manager, for her invaluable assistance. We would also like to thank the editorial team and the anonymous reviewers for their helpful comments.
Conflict of interest statement. None declared.
References
Coresh J, Astor BC, Greene T, et al. Prevalence of Chronic Kidney Disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey.
John R, et al. Unreferred chronic kidney disease: a longitudinal study.
De Vecchi AF, Dratwa M, Wiedermann ME. Healthcare systems and ESRD therapies – an international review. Costs and reimbursement/funding of ESRD therapies.
United States Renal Data System (USRDS). USRDS Annual Report (2003): incidence and prevalence of ESRD.
Roderick P, Davies R, Jones C, et al. Simulation model of renal replacement therapy: predicting future demand in England.
Wight JP, Oliver A, Payne N. A computer model for predicting the demand for end-stage renal failure (ESRF) treatment, contract setting and monitoring.
Briggs JD, Berthoux F, Jones E. Predictions for future growth of ESRD prevalence.
Vestergaard P, Lokkegaard H. Predicting future trends in the number of patients on renal replacement therapy in Denmark.
Xue JL, Jennie Z, Louis TA, et al. Forecast of the number of patients with end-stage renal disease in the United States to the year 2010.
Branley P, McNeil JJ, Stephenson DH, et al. Modelling the future dialysis requirements under various scenarios of organ availability in Australia.
Department of Health.
Anonymous: K/DOQI clinical practice guidelines for chronic kidney disease: evaluation classification and stratification. Kidney Disease Outcomes Quality Initiative.
Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study.
Vanholder RC, Massy ZA, Argiles A, Spasovski G, Verbeke F, Lamiere N. Chronic kidney disease as cause of cardiovascular morbidity and mortality.
Keith DS, Nichols GA, Gullion CM, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organisation.
Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease (the modification of diet in renal disease study).
Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and the progression of nondiabetic renal disease: a meta-analysis of patient-level data.
Kshirsagar AV, Joy MS, Hogan SL, et al. Effect of ACE inhibitors in diabetic and nondiabetic chronic renal disease: a systematic overview of randomized placebo-controlled trials.
Feest TG, Dunn EJ, Burton CJ. Can intensive treatment alter the progress of established diabetic nephropathy to end stage renal failure?
Rossing K, Christensen PK, Hovind P, Tarnow L, Rossing P, Parving HH. Progression of nephropathy in type 2 diabetic patients.
Jones C, Roderick P, Harris S, Rogerson M. An evaluation of a shared Primary and Secondary Care Nephrology Service for managing patients with moderate to advanced CKD.
The Joint Specialty Committee on Renal Disease of the Royal College of Physicians of London and the Renal Association.
Renal Association.
Levey AS, Bosch JP, Breyer Lewis J, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation.
Levey AS, Greene T, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine.
National Kidney Foundation.
Levey AS, Gassman JJ, Hall PM, Walker WG. Assessing the progression of renal disease in clinical studies: effects of duration of follow-up and regression to the mean.
Van Biesen W, Vanholder R, Veys N, et al. The importance of standardization of creatinine in the implementation of guidelines and recommendations for CKD: implications for CKD management programmes.
Coresh J, Eknoyan G, Levey AS. Estimating the prevalence of low glomerular filtration rate requires attention to the creatinine assay calibration.
Froissart M, Rossert J, Jacqout C, Paillard M, Houillier P. Predictive performance of the Modification of Diet in Renal Disease and Cockroft–Gault equations for estimating renal function.
Hallan S, Asberg A, Lindberg M, Johnsen H. Validation of the Modification of Diet in Renal Disease formula for estimating GFR with special epmhasis on calibration of the serum creatinine assay.
Lamb EJ, Tomson CRV, Roderick PJ. Estimating kidney function in adults using formulae.
Bostom AG, Kronenberg F, Ritz E. Predictive performance of renal function equations for patients with chronic kidney disease and normal serum creatinine levels.
Department of Health.
Department of Health.
Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology and management.
Perneger TV, Brancati FL, Whelton PK, Klag MJ. End stage renal disease attributable to diabetes mellitus.
He J, Whelton PK. Elevated systolic blood pressure as a risk factor for cardiovascular and renal disease.
Marcantoni C, Jafar TH, Oldrizzi L, Levey AS, Maschio G. The role of systemic hypertension in the progression of non-diabetic renal disease.
Iseki K, Ikemiya Y, Iseki C, et al. Proteinuria and the risk of developing end-stage renal disease.
UK Prospective Diabetes Study (UKPDS) Group: Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33).
Pohl MA, Blumenthal S, Cordonnier D, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the Irbesartan Diabetic Nephropathy Trial: clinical implications and limitations.
Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the Modification of Diet in Renal Disease Study.
Ruggenenti P, Perna A, Mosconi L, et al. Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. ‘Gruppo Italiano di Studi Epidemiologici in Nefrologia’ (GISEN).
Strippoli GFM, Craig M, Deeks JJ, Schena FP, Craig JC. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review.
Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes.
Viberti GC, Mogensen CE, Groop LC, Pauls JF. Effect of captopril on progresion to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria.
Ruggenenti P, Perna A, Gherardi G, et al. Renoprotective properties of ACE inhibition in non-diabetic nephropathies with non-nephrotic proteinuria.
Maschio G, Alberti D, Janin G, et al. Effect of the angiotensin-converting-enzyme-inhibitor benazepril on the progression of chronic renal insufficiency.
Bergstrom J, Alvestrand A, Bucht H, et al. Progression of chronic renal failure in man is retarded with more frequent clinical follow-ups and better blood pressure control.
Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood pressure control on the progression of chronic renal disease.
UK Prospective Diabetes Study Group: tight blood pressure control and risk of microvascular and macrovascular complications in type 2 diabetes: UKPDS 38.
Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group.
Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalisation.
Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all cause mortality: a pooled analysis of community based studies.
Shulman NB, Ford CE, Dallas Hall W, et al. Prognostic value of serum creatinine and effect of treatment of hypertension on renal function.
Flack J, Neaton J, Daniels B, et al. Ethnicity and renal disease: lessons from the Multiple Risk Factor Intervention Trial and the Treatment of Mild Hypertension Study.
Author notes
1Department of Public Health Sciences & Medical Statistics, University of Southampton, Level C(805), South Academic Block, Southampton General Hospital, Tremona Road and 2Department of Nephrology, D Level East Wing, Southampton General Hospital, Southampton, UK





Comments