“I cannot keep pace with my husband. Is it simply ageing, overweight and my sedentary lifestyle?” This is a common encounter in primary care, bearing in mind that around 16 % of older community-dwelling people experience at least grade 3 shortness of breath according to the Medical Research Council questionnaire (“walk slower than people of the same age because of breathlessness or have to stop for breath when walking at my own pace on the level”).1
Heart failure (HF) is a common syndrome, predominantly occurring in the elderly, with a significant impact on quality of life, high mortality rates and unplanned hospitalisations that place a significant burden on health care systems and budgets in developed countries.2 General practitioners (GPs) play an important role in the disease trajectory of a patient with HF. In particular, GPs have a pivotal role in the diagnostic and palliative phase, and participate in co-operative care with specialist teams in the intervening period.
Three important reasons underlie the gradual shift from hospital-based care to primary care being seen in many developed countries. First, in the last decade, heart failure with a preserved ejection fraction (HFpEF) is increasing, while the prevalence of heart failure with a reduced ejection fraction (HFrEF) is decreasing. For HFpEF, hospital care is in general not necessary, except in cases with acute exacerbations, and it is characterised by multiple comorbidities, which benefit from generalist care. A second reason is that governments are increasingly shifting chronic disease care to primary care, given international evidence on cost-effectiveness. Studies have shown that if HFrEF patients are adequately up-titrated, the care provided by GPs is as good as that of a HF clinic.3,4 A final reason is that risk stratification with natriuretic peptides and up-titration of cardiovascular (CV) drugs of high-risk people from the community, e.g. those with a previous coronary event, hypertension or type 2 diabetes, effectively reduces the development of HF and CV hospitalisations. Early initiation or up-titration of angiotensinconverting enzyme inhibitors (ACE-inhibitors), angiotensin receptor blockers (ARBs) and beta-blockers has been shown to be effective in this group.5,6
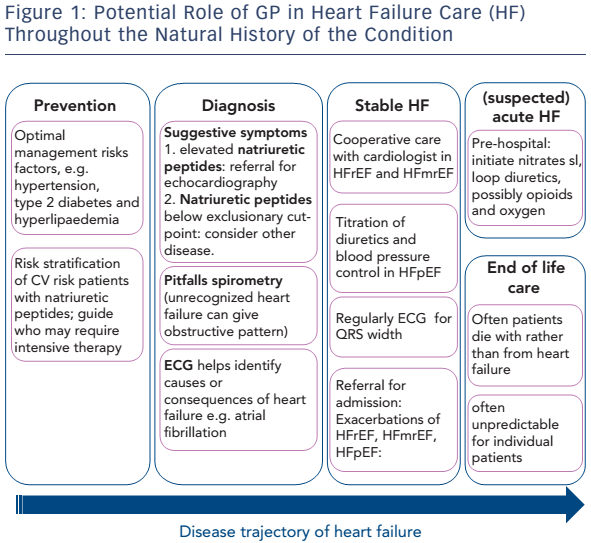
GPs should be prepared for this transition in care. Here, the authors give a framework for the potential role of GP in HF care throughout the natural history of the condition (see Figure 1).
Definition, Diagnosis, Case Finding and Risk Stratification
A diagnosis of HF requires a combination of clinical features – such as breathlessness, fatigue and ankle oedema – together with a structural or functional abnormality of the heart that impairs its ability to pump or relax on echocardiography.2,7 Pump failure may be caused by reduced contraction of the left ventricle, measured as a reduced ejection fraction (EF; <40 %). Reduced EF is almost always accompanied by impaired filling of the left ventricle, but in some patients reduced filling dominates whereas the EF is normal. This failure of relaxation of the heart in diastole and reduced filling is termed HF with preserved EF (≥50 %). 7 The updated European Society of Cardiology (ESC) guidelines on HF have recently introduced an ‘in-between’ category; HF mid-range EF (HFmrEF; EF 40–49 %), which typically has features of both HFrEF and HFpEF.2
HFrEF is best understood. It typically develops after myocardial infarction, when myocyte loss results in left ventricular (LV) dilatation and diminished contraction.7,8 HFpEF may develop after longstanding hypertension, but also in those with obesity and type 2 diabetes.8 Compensatory myocardial stiffening results in reduced filling capacity of the normal sized or even small left ventricle. This leaves a ventricle with an EF in the normal range but a reduced stroke volume.7 Patients with HFpEF may have particularly bothersome symptoms during exercise.7,8
Overdiagnosis and Underdiagnosis
Especially in the early stages, the detection of any type of HF is difficult because symptoms and signs are non-specific. Breathlessness, a key symptom of HF, may be confused with chronic obstructive pulmonary disease (COPD), obesity or deconditioning.7 Signs are mostly related to fluid overload and include elevated jugular venous pressure, pulmonary crackles and ankle oedema, but fluid overload may be absent, particularly in patients receiving diuretics for hypertension.2 A displaced or broadened/sustained apex beat is suggestive of HF, but palpation of the apex beat is infrequently performed nowadays in clinical practice.7 Fluid overload (backward failure) and compensation or adaptation of the heart (increased heart rate abnormal apex beat), or reduced oxygen delivery to metabolising tissues (forward failure) may result in symptoms or signs, but these may be hard to recognise, e.g. mild cognitive impairment, muscle fatigue or delayed recovery after exercise. Also, important possible causes (or consequences) of HF may be found on examination, e.g.a cardiac murmur. It should be noted that, especially in early HF, symptoms may be transient rather than present all the time. Other important aspects come from the patient’s history: ischaemic heart disease, particularly prior myocardial infarction, type 2 diabetes and hypertension should trigger the GP to ask for symptoms suggestive of HF.
These problems with key clinical features may lead to underdiagnosis, as was clearly shown by the high prevalence rates of unrecognised HF (constituting up to 80 % of all HF cases) in high-risk community populations, e.g. older people with breathlessness, type 2 diabetes or COPD from primary care.9–11 When these patients present to the GP, symptoms that could suggest HF may not be recognised as such or may be confused with other diagnoses, and also not reported by the patients themselves. Moreover, atypical presentation or comorbidities can complicate identification of HF. For instance, in a patient diagnosed with COPD, it may be unclear whether progression of shortness of breath is due to COPD or HF.12 Thus, case finding strategies in primary care yield a high proportion of patients with previously unknown HF, and notably HFpEF. This is an important issue when it comes to treatment (see below). On the other hand, overdiagnosis also exists, although this seems to be an issue of less significance in primary care with around 17 % incorrectly labelled with HF.13,14
Risk Stratification
Results from two randomised controlled trials (RCTs) provide us with encouraging results that a preventive strategy in high-risk CV patients may be effective.5,6 Both studies showed that ‘asymptomatic’ (or not mentioned symptoms spontaneously) type 2 diabetes patients, and people who have established CV risk factors or disease could be stratified with B-type natriuretic peptides (BNPs). Those with a BNP >50 pg/mL or N-terminal pro-B-type natriuretic peptide (NTproBNP) >125 pg/mL had positive outcomes if intensively treated with renin–angiotensin–aldosterone system (RAAS) inhibitors.5,6 In the St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial, this resulted in a reduction in LV dysfunction with or without HF; 5.3 % in the intervention arm compared with 8.7 % in the care as usual group (odds ratio 0.55, p=0.003). Also, the incidence rate of emergency hospitalisation for major CV events decreased by the intervention.5 In the Nt-proBNP Guided Primary Prevention of CV Events in Diabetic Patients (PONTIAC) trial in which patients were up-titrated to maximal tolerated renin–angiotensin system (RAS) inhibitors and beta-blockers, the primary endpoint hospitalisation/death due to cardiac disease at 2 years was significantly reduced compared with care as usual (hazard ratio 0.35 (95 % CI [0.13–0.98]; p<0.044).6
Respiratory Symptoms and Abnormal Spirometry
Symptoms such as breathlessness, cough and wheezing may not necessarily reflect pulmonary disease. HF, and as a result fluid in the lungs, can cause wheezing by the fluid compression from outside the bronchioles. In the case of asthma and COPD, the mainly expiratory obstruction comes from the muscular layer around the bronchioles, or from leucocyte accumulation and inflammation inside the bronchioles, giving the same symptomatology. It is therefore important to consider that wheezing can have a cardiac origin as well.
A similar pitfall should be acknowledged with respect to spirometry. In the presence of clinically detectable pulmonary fluid overload, e.g. pulmonary crepitations or ankle oedema, patients with (unrecognised) HF have a greater reduction in forced expiratory volume in 1 second (FEV1) than in the forced vital capacity (FVC).15 Since a diagnosis of COPD is based on ‘obstruction’ with spirometry, operationalised as FEV1/FVC <70 %, patients with (unrecognised) HF may remain undetected and can be labelled as COPD. Thus, overdiagnosis of COPD can occur at the cost of missing HF.15–17 Spirometry should therefore only be performed in stable and euvolemic patients, to prevent overdiagnosis of COPD.15
Additional Tests: Natriuretic Peptides,Electrocardiography and Imaging
When HF is suspected on the basis of medical history and signs and symptoms, additional diagnostic investigations are required to exclude HF, or select those who need further testing and identify whether the HF is associated with a reduced or preserved EF. Echocardiography can either be performed right away if a patient is known to have a prior myocardial infarction or atrial fibrillation (AF), or based on the result of natriuretic peptide assays and electrocardiography.2
For primary care, rather low exclusionary cut-off values for natriuretic peptide levels are recommended by the ESC guidelines on HF.2 These are set so that the likelihood of HF is low if values are below the cutoff point.2,18–23 Exclusionary cut-off values of <125 pg/mL for NTproBNP and BNP <35 pg/mL are recommended in the ESC guidelines,2 while the National Institute for Health and Care Excellence (NICE) in the UK recommends 400 pg/mL and 100 pg/mL, respectively.24 When applied in the primary care setting, 125 pg/mL for NTproBNP results in a negative predictive value of over 99 % at the cost of many more echocardiograms than a cut-off value of 400 pg/mL, which has a negative predictive value of about 97 %.18 Missed cases will mainly be those with HFpEF. 18 Using low cut-off points for non-acute patients suspected of HF is useful in the light of the lower a priori chance of disease and, most importantly, because of the milder severity of the disease than in acute breathlessness.2,18 It should be noted that in the case of non-acute breathlessness, other causes may underlie elevated NTproBNP levels, including AF, age over 75 years, renal impairment and LV hypertrophy, but not mild-to-moderate COPD.2,7
B-type natriuretic peptides (BNP and NTproBNP) are produced by myocytes in response to increased wall stress, which is in general lower in HFpEF than in HFrEF, in line with Laplace’s law (wall tension=pressure x radius/wall thickness).7 With similarly elevated LV pressures, the wall stress and thus production of B-type NPs is lower in HFpEF because the diameter of the ventricle is smaller and wall thickness higher (concentric remodelling), while in HFrEF the left ventricle is dilated and its wall thinned (eccentric remodelling). Moreover, B-type NPs may normalise in 24 hours and thus BNP and NTproBNP may be in the normal range when measured a day or more after the patient has visited the GP for symptoms of breathlessness during exercise.25,26
If a patient has natriuretic peptide levels above the cut-off values, echocardiography is usually indicated as the next step in the diagnostic process. Open access echocardiography is still not available to most primary care physicians. This could be a useful means to bring earlier diagnosis of HF to primary care. With echocardiography, HFrEF can be distinguished from HFpEF and HFmrEF. For HFrEF, the single measurement of a left ventricular ejection fraction (LVEF) <40 % is widely accepted (preferably seen in combination with a dilated left ventricle).2 For HFpEF, the description and ranges for abnormal parameters is still debated and is currently defined by expert consensus. Most consider a combination of measurements are needed, including:
- a (nearly) normal EF;
- left atrial enlargement;
- increased LV mass or wall thickness; and
- raised LV filling pressures.2,7
Assessing diastolic dysfunction is even more unclear as measuring LV filling non-invasively with echocardiography in particular is difficult.7
Other investigations, such as the electrocardiogram (ECG), chest X-ray and other blood tests other than natriuretic peptides might also be considered in the diagnostic work-up of a patient with possible HF. An ECG is useful to detect possible causes and consequences of HF, such as AF. Chest X-ray is not very helpful, unless in the case of clear fluid overload. In that situation, however, signs and symptoms generally already point in the same direction. Chest X-ray is not worthwhile for diagnosing COPD, but may help to diagnose or exclude pulmonary malignancy.2 Blood tests other than B-type NPs can be useful to rule out precipitating factors such as thyroid disease or anaemia, measure modifiable CV risk factors such as cholesterol and assess baseline liver and renal function prior to initiating treatment.
Overall, history taking and investigation of signs and symptoms is very important in primary care. Of additional tests, natriuretic peptides are most informative and valuable.18–20,23 High-risk patients (e.g. type 2 diabetes, COPD) may benefit from risk stratification based on B-type NPs and case finding.
When to Refer a Patient
The decision to refer a patient will depend on the individual expertise of the GP and the organisation of the health care system. Most guidelines advocate an initial specialist assessment to make the formal diagnosis of HF. Once a definitive diagnosis is reached, specialists may initiate HF medication or this may be done by the GP. Consideration of device therapy is usually done at the specialist level based on parameters including EF and widening of the QRS on the ECG. Referral for rehabilitation may also be via specialist or GP teams.
Prevention of Heart Failure
The 2013 European Society of Hypertension/ESC guidelines for the management of arterial hypertension state that hypertension is the most important attributable risk factor for developing HF.27,28 Preventing HF is the largest benefit associated with blood pressurelowering drugs. This was seen in treatment with diuretics, betablockers, ACE inhibitors and ARBs.29 The elderly are no exception.30 Thus, adequately addressing high blood pressure in primary care is important to prevent development of HF. Optimal treatment of other CV risk factors such as hypercholesterolaemia and type 2 diabetes through pharmacological and lifestyle interventions is also important to prevent HF. Timely management of myocardial infarction to reduce muscle loss may also help to reduce the number of patients developing LV dysfunction in the longer term.
What is the Prevalence of Heart Failure?
Although, the ESC guidelines on HF mention a prevalence of 1–2 % in the general population, a recent systematic review suggests that it is closer to 4 %.2,31 Reports based on hospitalised patients suggest that around 50 % of the patients have HFpEF and 50 % have HFrEF, with a time trend towards an increase in HFpEF.32,33 Population prevalence data among adults aged 65 years or over living in the community with HF found that around 75 % had HFpEF and 25 % had HFrEF.34
What is the Prognosis?
The prognosis of HF, a chronic progressive disease, depends on the point in time the diagnosis is made (early or late in the disease trajectory) and thus the severity of the disease. Around one in 10 will have died five years after diagnosis, rising to around one in three for cases first detected during hospitalisation. 7,35,36 As a comparison, the five year mortality of colon cancer is around one in three.
How Should Heart Failure be Managed?
Lifestyle interventions, pharmacological therapies, devices and multidisciplinary disease management programmes can relieve symptoms, improve prognosis and optimise quality of life of patients with HFrEF.2 For HFpEF, clear mortality-reducing drugs are not yet available. Based on expert opinion, it is recommended to use loop diuretics to keep the patient euvolemic with careful titration, encourage aerobic exercise, optimise blood pressure control and control of heart rate in AF.2,37 From a health care system viewpoint, it is also important to manage costs, which can particularly be achieved by preventing hospital admissions where possible.38
It is vital that patients with HF, and their carers where applicable, understand their condition and are actively involved in management decisions, and to encourage self-care. eHealth strategies may be supportive in this regard. Patients should be encouraged to avoid overuse of salt, follow a healthy diet, adhere to prescribed drugs and do regular exercise. For patients with more advanced HFrEF, interventions such as daily weighing and fluid restriction (<1,500 mL/day) may be required. In patients with an EF <35 % after optimising therapy, an implantable cardioverter-defibrillator (ICD) may be considered to prevent sudden death. In those with an EF <35 % and QRS duration >130 ms, cardiac resynchronisation therapy might be considered by the cardiologist.2 Pharmacological therapies are the mainstay of treatment in HF and will be more closely considered below.
Pharmacological Treatment
Loop diuretics are essential to relieve symptoms, particularly in acute situations, for HFrEF, HFmrEF or HFpEF. Most patients once stabilised still require ongoing diuretics, although often at a lower dosage. These are the only drugs that can adequately remove fluid from the body in those with congestion.
In those with HFrEF, up-titration of ACE inhibitors (or ARB when ACE inhibitors are not tolerated; dry cough in up to 5 % of cases) and beta-blockers should follow. Additionally, mineralocorticoid receptor antagonists (MRAs), such as spironolactone or eplerenone can also be added in patients who remain symptomatic.2 Diuretic use may be temporarily discontinued or reduced if patients are up-titrated with RAS inhibitors and beta-blockers, but the majority of patients will need to continue taking diuretics at some level. If after this combination of therapies, patients still have symptoms, or have a LVEF <35 % and a broad QRS complex on the ECG, and/or a LVEF <30 %, further management should be done by the cardiologist (such as the fitting of cardiac synchronisation devices or ICD).
If symptoms (New York Heart Association [NYHA] class II–IV) still persist despite the use of these three drugs plus diuretics, some patients may benefit from ivabradine, which slows heart rate through a mechanism independent of beta-blockers, if they are in sinus rhythm, LVEF <35 % and their heart rate is >70 beats/min.2
As already mentioned, patients with HFpEF benefit from adequate titration of diuretics, which can give important symptom relief.2
Drugs acting on the RAS that yield good results in HFrEF, such as ACE inhibitors and ARBs, have not shown clear benefits in HFpEF. 39–41 MRAs, however, may play a role in the future. In the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial, the MRA spironolactone was compared with placebo. Overall, it did not reduce the incidence of the composite of death from CV causes, aborted cardiac arrest or hospitalisation for the management of HF (HR 0.89; 95 % CI [0.77–1.04]), but it did lower the incidence of first and total number of HF hospitalisations.
Also, an interaction by inclusion stratum and region was seen, with no favourable effects in Russia/Georgia (HR 1.10; 95 % CI [0.79–1.51]), but a favourable profile was seen on the primary outcome in the US, Argentina and Brazil (HR 0.82; 95 % CI [0.69–0.98]) where most included patients that had an elevated natriuretic peptide level at inclusion.42 Thus, the TOPCAT trial does not give conclusive evidence on the use of spironolactone in HFpEF, but it may hint at a benefit for patients with elevated natriuretic peptide levels.
Novel Treatment Option
A new drug class has recently emerged for the management of patients with HF with reduced EF. The so-called angiotensin receptor neprilysin inhibitor (ARNI) class currently has one drug available for clinical use. Sacubitril-valsartan (formerly known as LCZ696), exerts a dual action; it consists of an ARB (valsartan) and a neprilysin inhibitor (which inhibits the enzyme that breaks down active BNP). It acts to reduce sympathetic tone, aldosterone levels and sodium retention, through inhibition of the overactive RAS by an ARB while simultaneously potentiating the effect of the protective vasoactive neuropeptide BNP through neprilysin inhibition.
Sacubitril-valsartan (formerly known as LCZ696), was evaluated in the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial in comparison with enalapril 10 mg twice daily.43 After 27 months of follow-up, the trial was stopped early due to positive interim results. In symptomatic patients with HFrEF (LVEF <40 %, BNP >150 pg/mL, mean age 63.8 years), who were treated with ACE inhibitor or ARB, and other background HF therapies such as beta-blockers and MRAs, the absolute risk of the composite of CV mortality and hospitalisation for HF was reduced by 4.7 % (21.8 versus 26.5 %, relative risk reduction 20 %) with sacubitril-valsartan versus enalapril in HFrEF patients on optimal HF background therapy. All-cause mortality was 17.0 % with the ARNI as compared with 19.8 % with enalapril, yielding a hazard ratio of 0.84 (95 % CI [0.72–1.31]; p<0.001).43
It should be noted that relative to primary care practice, included patients were relatively young and 21 % were female. Moreover, as a consequence of a run-in phase in the trial design, only patients who could tolerate ACE inhibitor and ARB were enrolled. Indeed, not many adverse effects were reported.
Data from the Management of Heart Failure with Preserved Ejection Fraction (PARAMOUNT) trial comparing sacubitril-valsartan with valsartan in HFpEF, showed that ARNI reduced NT-proBNP levels, left atrial volume index and increased the estimated glomerular filtration rate (eGFR), more so than with valsartan alone, independent of its systolic blood pressure-lowering effect.44 The potential benefit of an ARNI in HFpEF is investigated further in the ongoing Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients with Preserved Ejection Fraction (PARAGON) trial.
Sacubitril-valsartan may not be prescribed by GPs in the following years, but this may change in the future.
Renal Function
Managing patients with HF requires careful monitoring and prescribing. In particular, balancing the use of (loop) diuretics and their adverse effect on kidney function can be challenging. To help safely manage these patients, prerenal dysfunction should be distinguished from post renal dysfunction. In prerenal dysfunction, the patient is dehydrated, due to the use of too high a dosage of diuretics, which causes the blood pressure in the kidney to be too low to filtrate. On the other hand, in post-renal dysfunction, there is too much fluid (venous congestion) and consequently too much venous pressure on the kidney. Both situations lead to decreased kidney function, more so with venous congestion. Practically, this means that patients who are overloaded should receive diuretics and this can have a beneficial effect on kidney function. GFR may even increase as a result. Caution is needed where the patient is already on diuretic treatment – giving too high a dose can lead to kidney under-perfusion and thus further deterioration in renal function. Urea and GFR together with a check of the patient’s fluid status by physical examination using parameters such as postural drop in blood pressure can help to monitor and adjust diuretic dosing. It is important to ensure that patients are not on other medications, which can adversely affect renal function and decrease effectiveness of diuretics such as nonsteroidal anti-inflammatory drugs.
Acute Heart Failure
When a patient presents with acute shortness of breath, high respiratory rate and lung crackles, immediate hospitalisation is required. However, in such suspected acute HF (AHF) cases, the following steps can be considered by the GP before the ambulance arrives, but only if they have the equipment, expertise and feel confident to do so:
- When systolic blood pressure >110 mmHg: sublingual nitroglycerin for immediate relief of breathlessness through venodilatation.
- Furosemide 40 mg intravenously (iv), and in those already on a loop diuretic an even higher dosage may be used, while awaiting the ambulance and the cardiologist (note that furosemide needs about 20 minutes to work).
- When oxygen saturation <92 %: titrate oxygen administration to achieve an oxygen saturation >92 %. This can be vital for immediate survival, but should not be provided routinely in those with oxygen saturations >92 %. This is because oxygen administration can cause vasoconstriction and a reduction in cardiac output. In COPD, hyperoxygenation may also increase the ventilation–perfusion mismatch, suppressing ventilation leading also to hypercapnia.
- When severely agitated: 5 mg morphine slowly iv can help reduce dyspnoea through venodilatation.
- Some pre-hospital systems may use continuous positive airways pressure to help improve oxygenation and reduce respiratory distress.
Heart Failure, Non-cardiac Comorbidities and Atrial Fibrillation
GPs have a particularly important role in overseeing the overall health status of patients. They are the physicians most aware of non-cardiac comorbid conditions. Treating such co-morbidities may improve HF symptoms. It should be noted, however, that effects of improving symptoms compared with improving prognosis may need to be carefully balanced. For instance, the Treatment of Predominant Central Sleep Apnoea by Adaptive Servo Ventilation in Patients with Heart Failure (SERVE-HF) trial showed that addressing central sleep apnoea, which is common in HFrEF with mask ventilation improved symptoms, but prognosis was worse.45
Importantly, cardioselective beta-blockers may be prescribed in patients with comorbid COPD. In those with comorbid type 2 diabetes, metformin is the preferred drug. Recently, the BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) showed that empagliflozin (an inhibitor of sodium-glucose cotransporter [SGLT-2] in the kidney) added to metformin for glucose-lowering had beneficial prognostic CV effects (CV mortality, non-fatal myocardial infarction and non-fatal stroke) compared with placebo in patients with diabetes and CV disease.46 Subgroup analysis has suggested that the benefit was consistent for patients with and without HF.47 AF and HF are both common in older people and often co-occur. Up to 50 % of those with AF may have HF, and because B-type NP is also elevated by AF itself, echocardiography should be considered to detect or exclude co-existing HF in those presenting with AF. In patients with HFrEF and AF, a recent individual patient data (IPD) meta-analysis of landmark HFrEF trials with betablockers showed that in general, those with HFrEF and AF seem not to benefit, while those in sinus rhythm do.48 Further research will provide us an answer whether this effect is related to the heart rate achieved with beta-blockers in those with HFrEF and AF.
Organisation of Care
Various examples of co-operative care have been developed across Europe. Often patients with HFrEF are managed in the hospital outpatient clinic for 3–6 months after diagnosis, to titrate medication to optimal doses. Hospital and community-based HF nurses can play an invaluable role in management and education of patients.
Although current guidelines recommend outpatient follow-up in specialised HF clinics, the optimal duration of these programmes has not been established, nor whether all or only high-risk patients would benefit. The randomised Danish NorthStar trial compared extended follow-up of stable patients on optimal medical therapy in the HF clinic with referral back to the GP.3 After a median follow-up of 2.5 years, no differences were seen in time to death or hospital admission with a CV problem (HR 1.17; 95 % CI [0.95–1.45]; p=0.149 HF outpatient clinics versus GPs), nor in any of the secondary outcomes of mortality, HF admission, quality of life, number of days admitted and number of admissions.3 Also, high-risk patients, as identified by NT-proBNP >1,000 pg/mL did not benefit from follow-up in a HF clinic, as compared with referral to their GP.3
The Dutch Comparative Study on Guideline Adherence and Patient Compliance in Heart Failure Patients (COACH-2) study also found no difference between follow-up in primary care versus in a HF clinic, in the number of deaths and CV hospital admissions. Guideline adherence was assessed by the guideline adherence indicator (GAI-3) as well as patient adherence (medication possession ratio [MPR]), and no differences were observed after 12 months.4 Both studies conclude that HFrEF patients can be referred back to primary care after initial management in hospital. The COACH-2 study group points out that, given the complexity of the HF syndrome and its comorbidities, close collaboration between health care providers is crucial to provide optimal, integrated care.
The Role of the General Practitioner in End-of-Life Care
Special attention should be dedicated to the last phase of life of HF patients. In a Dutch study, most elderly patients with HF (mean age 82.3 years) did not visit the cardiology outpatient clinic frequently in their last year of life (0.4 times), while home visits by the GP were more important (12.1 visits in last year).49 Of note, in the Netherlands, most (55.9 %) HF patients passed away at home or in a home for the elderly. Among those who died in hospital (32.6 %), only a small part died on the cardiology ward (5.8 % of total). Thus, most patients die with, not of, HF. Causes of death in this study were sudden death (28 %), progressive HF (23 %), cancer (20 %) or other causes (29 %).49
It is important to realise that there is tremendous individual variation in the disease trajectory of HF. One cannot know when the palliative phase starts; patients generally do not follow a gradual downward path. Some feel and function quite well and die suddenly, while others may follow an upward path after a period of poor quality of life. Diverse and multiple comorbidities further complicate the disease trajectory, warranting regular monitoring. Thus, the GP plays a crucial role and should lead the end-of-life care of patients with HF.
Conclusion
HF is a complex condition of increasing prevalence that requires the input of GPs as well as specialist services to ensure patients receive best care across the disease spectrum, from prevention to end-of-life care.